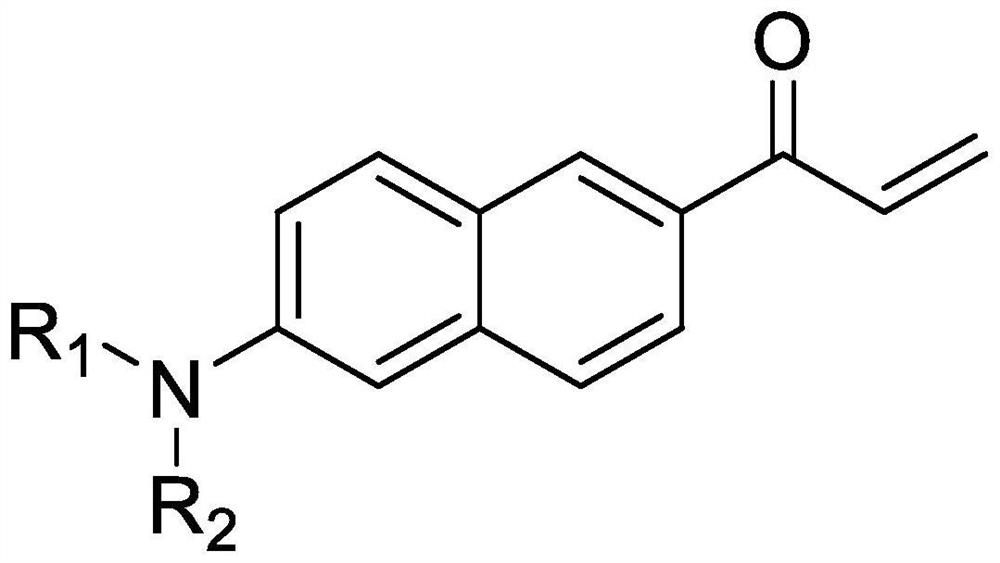
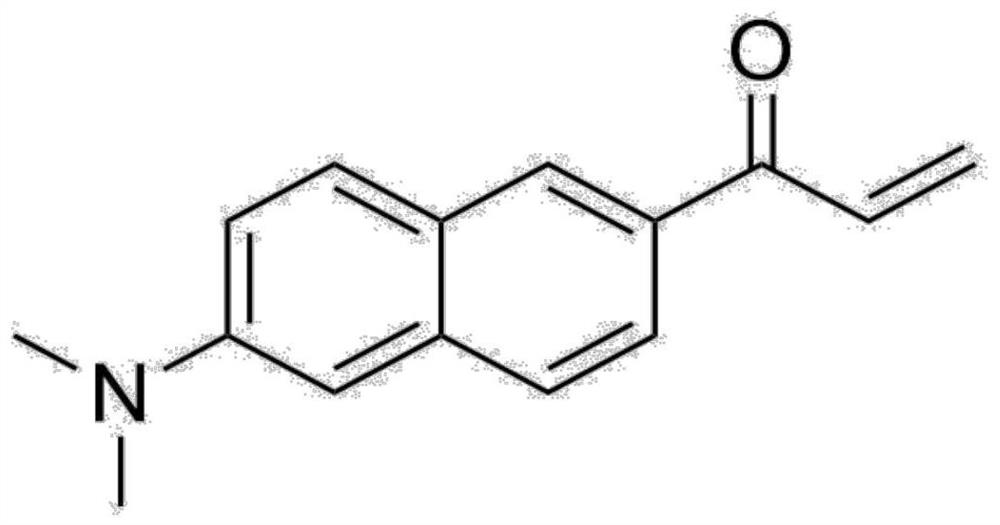
Synthesis method of Acrylodan and analogues thereof
A technology of acryloyl dan and a synthesis method, which is applied in the field of fluorescent probe synthesis in biochemistry, can solve the problems of expensive raw materials, many synthesis steps, complicated operations, etc., and achieves simple and easy operation, unique application prospect, and cheap and easy-to-obtain raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] (1) Compound 3-2 was synthesized by using 6-methoxy-2-acetylnaphthalene as a raw material to undergo substitution reaction with lithium dimethylamide.
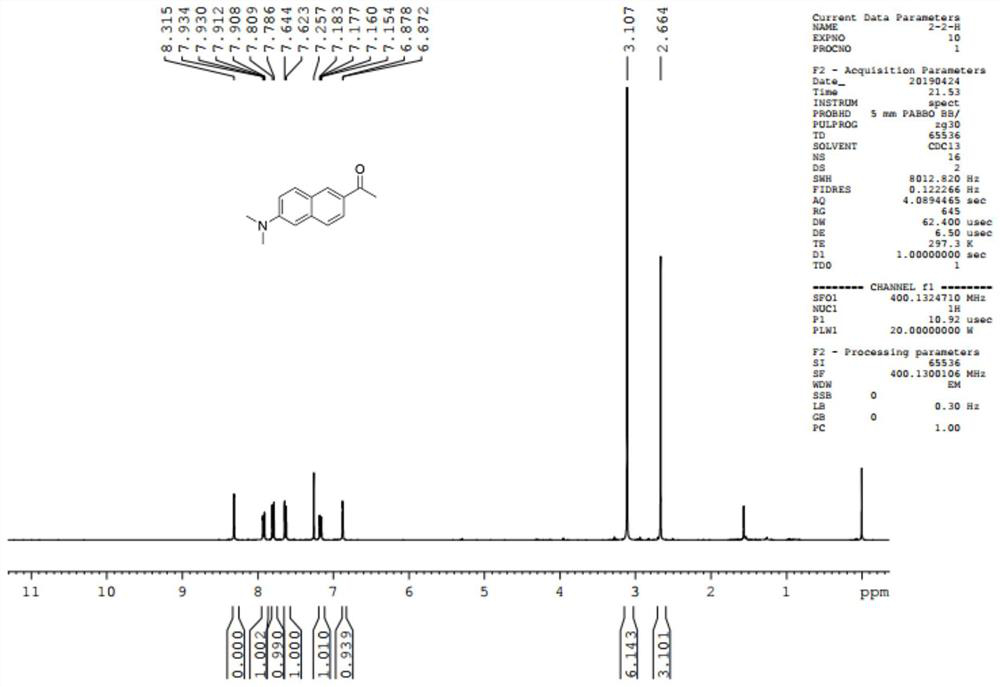
[0044] Operation method: Dissolve 6-methoxy-2-acetylnaphthalene (1.0g, 5mmol) into 5mL of anhydrous N,N-dimethylformamide, add 5mL of N,N-dimethylpropenylurea, LiN(CH 3 ) 2 solution (30mmol), the reaction was reacted at room temperature for 24 hours, after the reaction was completed, the reaction solution was added to ice water, concentrated hydrochloric acid was added to adjust the reaction solution to pH 1-2 range, extracted with ethyl acetate, the aqueous phase was collected, and then added to the solution. Solid sodium hydroxide was added to the aqueous phase to make it basic, extracted with ethyl acetate, the organic phase was collected, dried over anhydrous sodium sulfate, concentrated, and subjected to column chromatography to obtain compound 3-2 in a yield of 81%. 1 H NMR (400MHz, CDCl 3 )δ: 8.31(s, 1H), 7.93...
Embodiment 2
[0050] (1) Compound 3-2 was synthesized by using 6-methoxy-2-acetylnaphthalene as a raw material to undergo substitution reaction with lithium dimethylamide.
[0051] Operation method: 6-methoxy-2-acetylnaphthalene (1.0 g, 5 mmol) was dissolved in 5 mL of anhydrous tetrahydrofuran, 5 mL of acetamide was added, and LiN (CH) was added under the protection of argon. 3 ) 2 solution (30mmol), the reaction was reacted at room temperature for 24 hours, after the reaction finished, the reaction solution was added to ice water, concentrated hydrochloric acid was added to adjust the reaction solution to pH 1-2 range, extracted with ethyl acetate, the water phase was collected, and then added to the solution. Solid sodium hydroxide was added to the aqueous phase to make it basic, extracted with ethyl acetate, the organic phase was collected, dried over anhydrous sodium sulfate, concentrated, and subjected to column chromatography to obtain compound 3-2 in a yield of 81%.
[0052] (2) Co...
Embodiment 3
[0057] (1) Compound 3-2 was synthesized by using 6-methoxy-2-acetylnaphthalene as a raw material to undergo substitution reaction with lithium dimethylamide.
[0058] Operation method: Dissolve 6-methoxy-2-acetylnaphthalene (1.0 g, 5 mmol) into 5 mL of anhydrous tetrahydrofuran, add 5 mL of N,N-dimethylpropenyl urea, and add LiN (CH) under argon protection 3 ) 2 Solution (30mmol), the reaction was reacted at room temperature for 24 hours, after the reaction, the reaction solution was added to ice water, concentrated hydrochloric acid was added to adjust the reaction solution to pH 1-2, extracted with ethyl acetate, the water phase was collected, and then added to the solution. Solid sodium hydroxide was added to the aqueous phase to make it basic, extracted with ethyl acetate, the organic phase was collected, dried over anhydrous sodium sulfate, concentrated, and subjected to column chromatography to obtain compound 3-2 in a yield of 81%.
[0059] (2) Compound 3-3 is synthesi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com