Methods of treating lung cancer with a pd-1 axis binding antagonist, a platinum agent, and a topoisomerase ii inhibitor
A technology of topoisomerase and PD-L1, applied in chemical instruments and methods, antibody medical ingredients, medical preparations containing active ingredients, etc., can solve problems such as poor survival prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0459] The present disclosure will be understood in more detail with reference to the following examples. However, they should not be construed as limiting the scope of the invention. It should be understood that the examples and embodiments described herein are for illustrative purposes only, and various modifications or changes in view thereof will be suggested to those skilled in the art, and will be included within the spirit and scope of the application and the appended claims within the range.
example 1
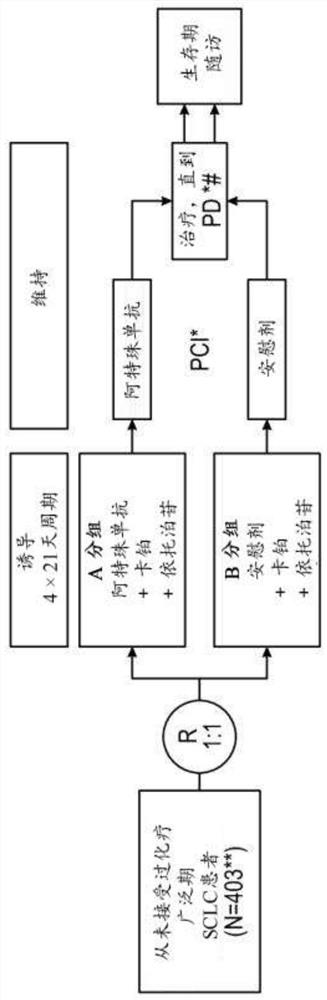
[0460] Example 1: Carboplatin plus etoposide with or without atezolizumab (anti-PD-L1 antibody) in untreated patients with extensive-stage small cell lung cancer (ES-SCLC) in phase I / III, randomized, Double-blind, placebo-controlled study,
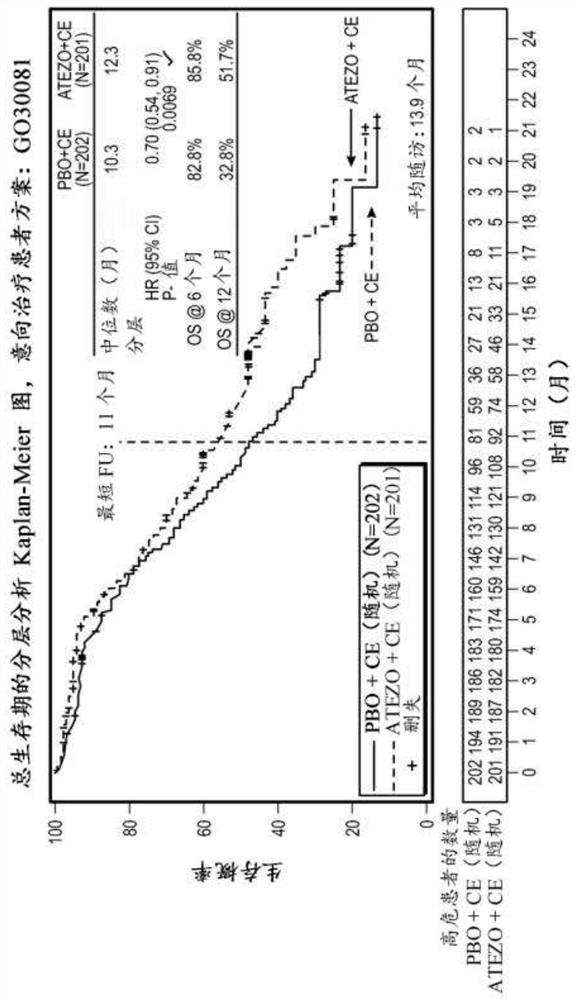
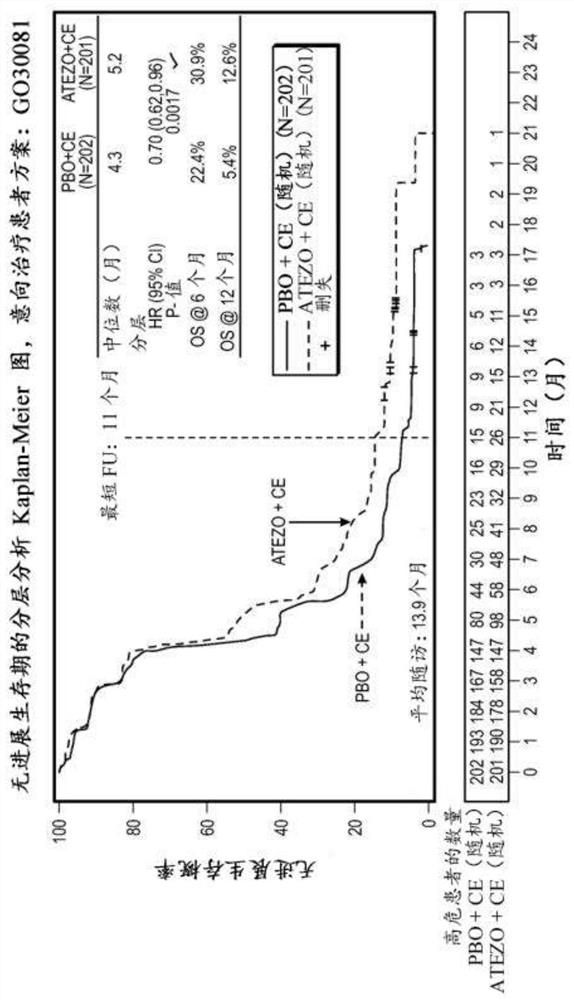
[0461] This study was designed to evaluate, compared with placebo, carboplatin, and etoposide, in chemotherapy-naive patients with ES-SCLC, when atezolizumab was given in combination with carboplatin and etoposide, the Whether antitumor effects in patients treated with atezolizumab would translate into statistically significant and clinically relevant improvements in PFS and OS. This study could assess the efficacy of atezolizumab in the ITT population and could assess exploratory immunization endpoints (such as but not limited to retrospectively assessed by PD-L1 expression) and their relationship to patient outcomes.
[0462] Research objectives
[0463] The primary efficacy goals of this study were as follows:
[0464] Evaluate the...
example 2
[0592] Example 2: Patient Reported Outcomes (PROs) from Example 1
[0593] Patients participating in the study described in Example 1 completed the European Organization for Research and Treatment of Cancer's Quality of Life Questionnaire C30 (EORTC QLQ-C30) and Quality of Life Questionnaire LC13 (QLQ-LC13) at baseline and every three weeks thereafter. Analysis included change from baseline, cumulative distribution function curves for change to week 12, and time to deterioration (TTD). Clinical significance is based on a change from baseline of ≥10 points (score range 0 to 100).
[0594]Completion rates were ≥85% at baseline and ≥70% at week 75 in both arms of the study. Baseline patient-reported outcome scores were comparable between the two groups. Patients in both cohorts reported early, significant improvement in symptoms, with numbers tending to be greater for Atezo+CE compared to placebo+CE. See Table 11. By week 12, a higher proportion of patients receiving Atezo+CE...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com