Antibody with double mic binding activity and application thereof
A technology that combines activity and antibodies, applied in the field of proteins, can solve problems such as instability and weak binding force, and achieve the effect of good affinity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 Preparation of membrane-expressed MIC protein and animal immunization
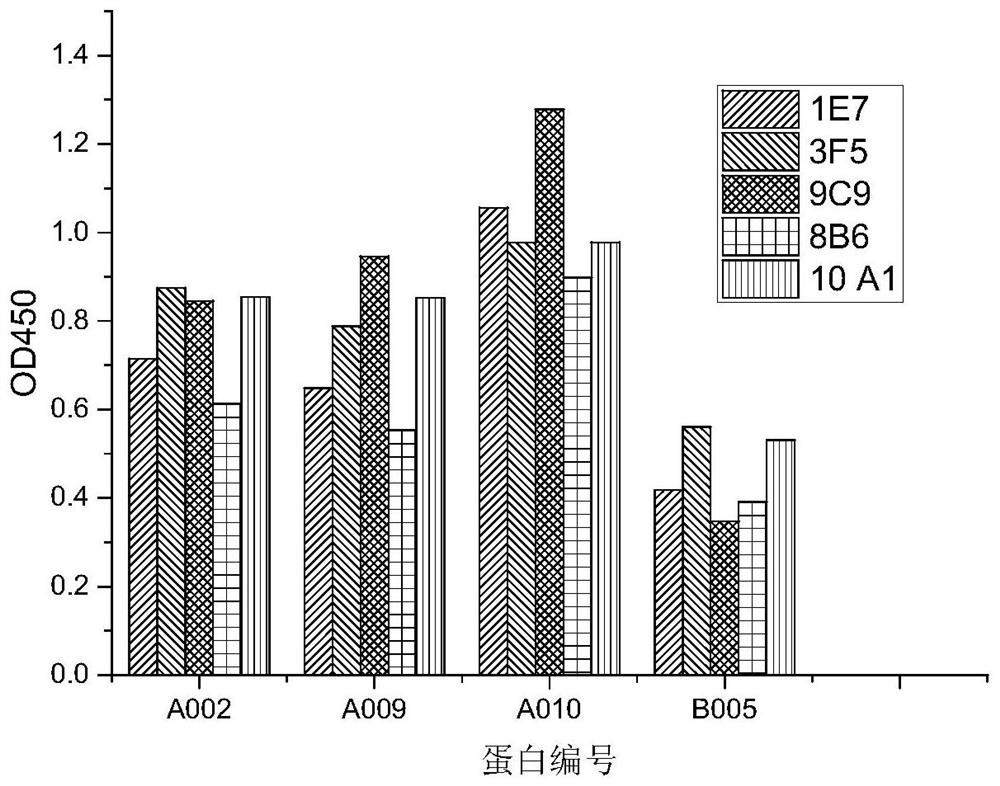
[0066] The full-length cDNAs of MICA*002, MICA*008, MICA*009, MICA*010 and MICB*005 will be expressed (see http: / / hla.alleles.org / alleles / text_index.html for the full-length sequence of MICA Contains signal peptide, amino acid sequence 1-366; extracellular region sequence contains signal peptide, amino acid sequence 1-284. MICB full-length sequence contains signal peptide, amino acid sequence 1-360; extracellular region sequence contains signal peptide, amino acid sequence 1- 286) was connected to the lentiviral vector pCDH-CMV-MCS-EF1-Puro, and then transferred into 293T cells together with the helper plasmid. The 293T cells were used to produce lentivirus, and the virus supernatant was collected to infect CHO cells. Take 5*10 6 3 transfected cells (5 types of cells with equal content) were mixed with complete Freund's adjuvant, shaken and mixed, and injected subcutaneously in the abdome...
Embodiment 2
[0067] Example 2 Preparation of secreted MIC protein
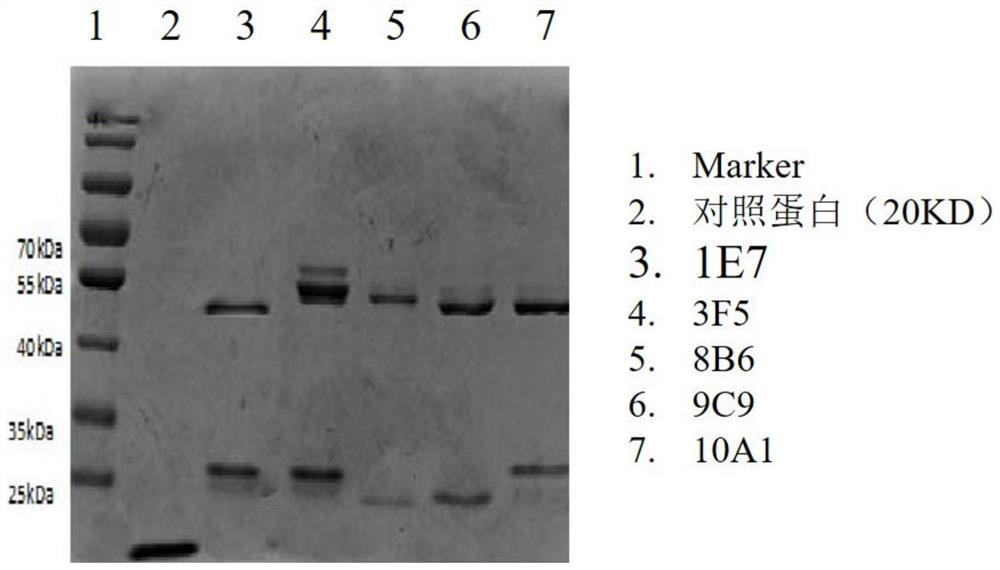
[0068] Use the cDNA of the extracellular region of MICA*002, MICA*008, MICA*009, MICA*010 and MICB*005 (the C-terminal his tag is designed) to connect with the lentiviral vector pCDH-CMV-MCS-EF1-Puro, and the auxiliary The plasmids were transferred together into 293T cells to produce virus, and the virus supernatant was collected to infect CHO cells. The CHO cell culture fluid was collected and purified with metal ion chelating filler to obtain the target protein. The molecular weight of MICA protein is about 55KD, and the molecular weight of MICB protein is about 45KD.
Embodiment 3
[0069] Example 3 Screening of monoclonal antibody cell lines
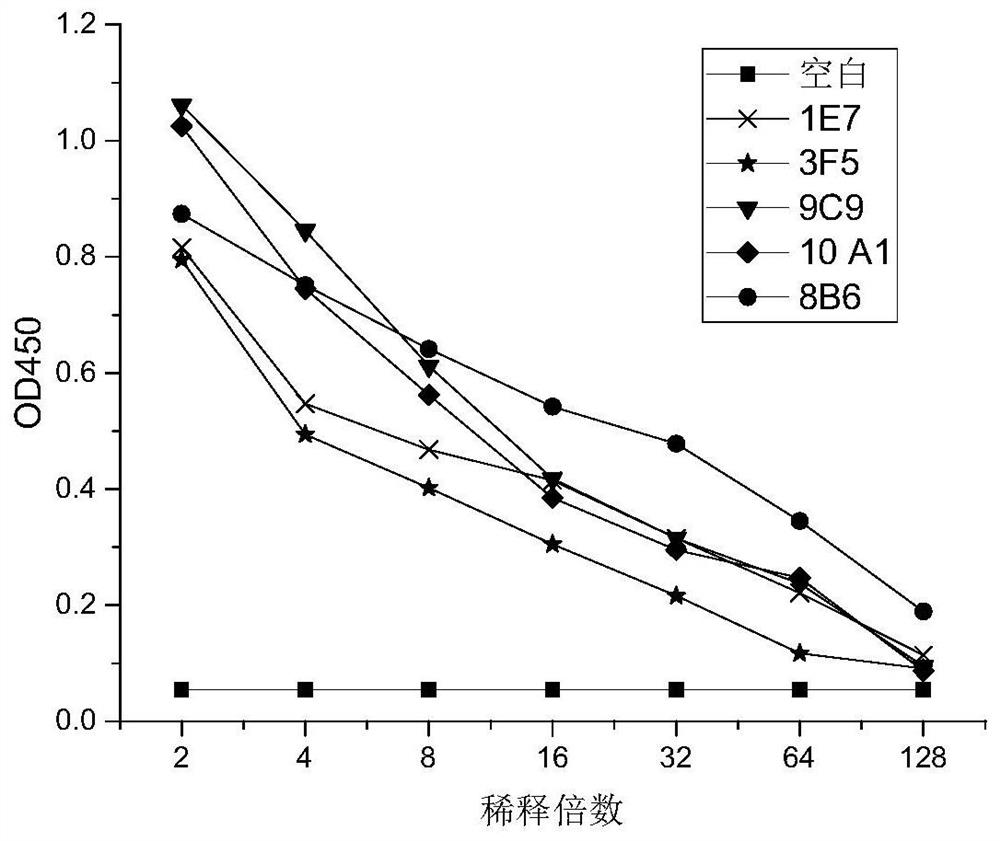
[0070] A total of 30 monoclonal cell lines were screened, and the monoclonal cells were picked up under a microscope, inoculated into 96-well plates, and placed in 37°C, 5% CO 2 After culturing in an incubator for 3 days, the supernatant was collected. The ELISA plate was coated with natural MICA*008, 100ng / well, incubated overnight at 4°C, and the primary antibody was 100µL / well of serially diluted cell culture supernatant (respectively diluted 2 times, 4 times, 8 times, 16 times, 32 times with PBS) , 64 times, 128 times), incubate at 37°C for two hours, secondary antibody: goat anti-mouse antibody (HRP labeled) diluted 1:5000, 100µL / well, incubate at 37°C for 1h, use TMB chromogenic solution for 10min, 450nm Detect absorbance. The five cell lines screened with higher binding ability to MICA*008 are 1E7, 3F5, 8B6, 9C9, and 10A1 cell lines, and the results are shown in figure 1 .
[0071] The anti-MIC antibodie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com