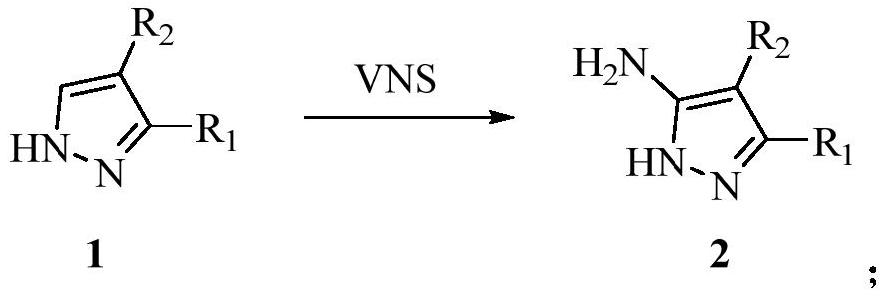
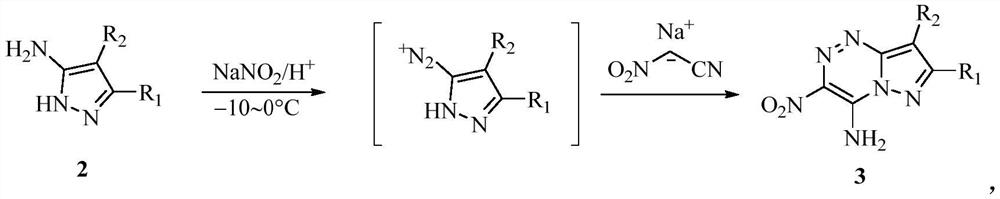
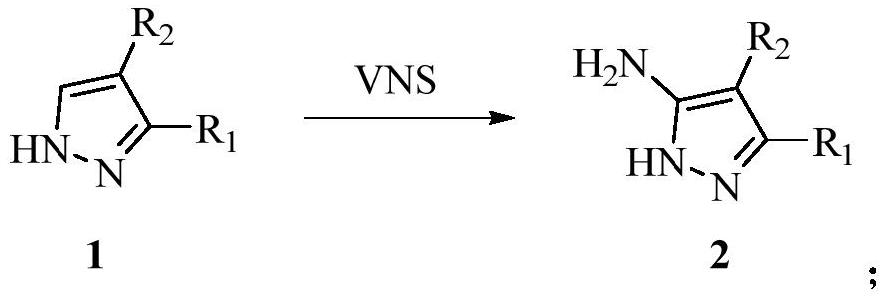
Synthesis method of pyrazolotriazine energetic compound
A synthesis method and technology of triazines, which are applied in the field of synthesis of pyrazolotriazine energetic compounds, can solve problems such as difficult application in actual production, insufficient reaction conditions, and high toxicity of reagents, and achieve low price, The effect of mild synthetic conditions and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Synthesis of 5-amino-3,4-dinitro-1H-pyrazole
[0031]
[0032] 3,4-Dinitro-1H-pyrazole (1g, 6.33mmol) was dissolved in dimethylsulfoxide (30mL), potassium tert-butoxide (2.84g, 2.53mmol), 4-amino- 4H-1,2,4-triazole (ATA) (1.28 g, 1.52 mmol). The reactant was stirred at 50°C for 8 h, extracted with ethyl acetate, washed with saturated NaCl solution, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to obtain crude 5-amino-3,4-dinitro-1H-pyrazole.
[0033] (2) Synthesis of 4-amino-3,7,8-trinitropyrazole-[5,1-c][1,2,4]triazine
[0034]
[0035] At 0°C, add 20 mL of 10% H to the crude 5-amino-3,4-dinitro-1H-pyrazole 2 SO 4 , and NaNO dissolved in 2.4 mL of water 2 (240 mg, 3.48 mmol) solution was added dropwise to the cooled dispersion, and the mixture was stirred for 2 h to form the corresponding diazonium salt in situ. NaOH (783 mg, 19.58 mmol) and nitroacetonitrile (538 mg, 6.25 mmol) were dissolved in 6 mL of ice water to pre...
Embodiment 2
[0039] Synthesis of 3-nitropyrazolo[5,1-c][1,2,4]triazin-4-amine
[0040]
[0041] The preparation method is the same as that in Preparation Example 1, except that the pyrazole derivative in step (1) uses pyrazole, and finally synthesizes 3-nitropyrazolo[5,1-c][1,2,4]tri The yield of azin-4-amine was 37%.
[0042] 1 H NMR (500MHz, DMSO-d 6 )δ9.48(s,2H),8.35(s,1H),7.11(s,1H)ppm.
[0043] 13 C NMR (126MHz, DMSO-d 6 )δ105.91, 130.69, 138.84, 140.51, 145.32ppm.
Embodiment 3
[0045] Synthesis of 7-methyl-3-nitropyrazolo[5,1-c][1,2,4]triazin-4-amine
[0046]
[0047] The preparation method is the same as in Preparation Example 1, except that the pyrazole derivative in step (1) is 3-methyl-1H-pyrazole, and finally synthesizes 7-methyl-3-nitropyrazolo[5, The yield of 1-c][1,2,4]triazin-4-amine was 43%.
[0048] 1 H NMR (500MHz, DMSO-d 6 )δ9.34(s,2H),7.91(s,1H),2.23(s,3H)ppm.
[0049] 13 C NMR (126MHz, DMSO-d 6 )δ21.13, 101.84, 129.52, 136.24, 141.79, 149.28ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com