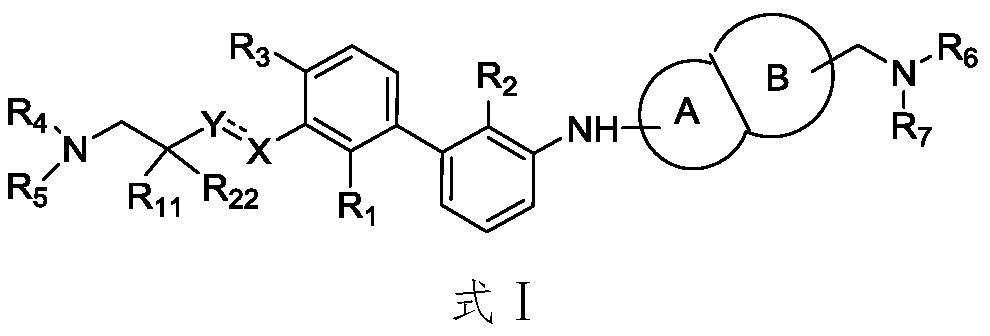
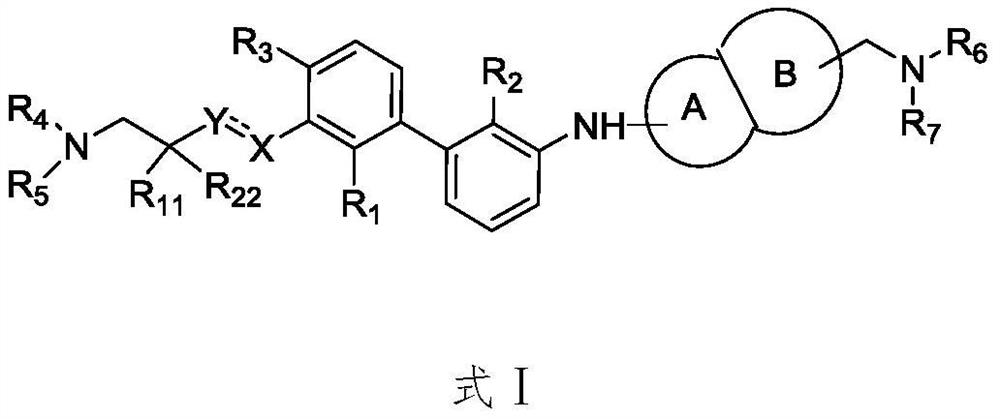
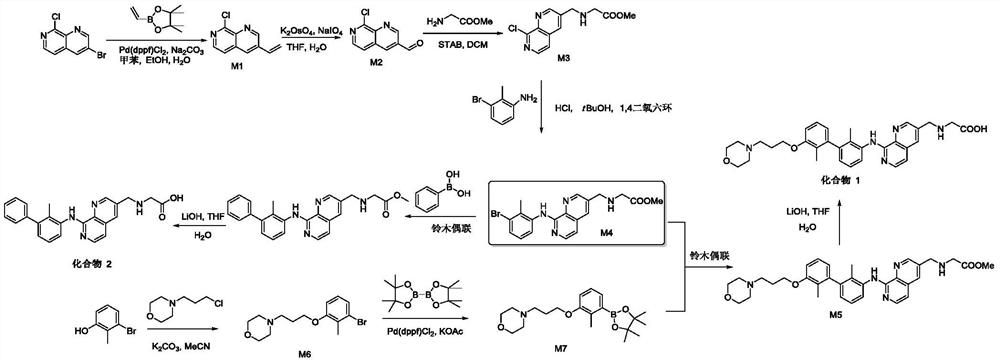
Immunomodulators, compositions and methods thereof
A compound and chelate technology, applied in the direction of drug combination, active ingredient of heterocyclic compound, pharmaceutical formulation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0171] The synthesis of embodiment 1 compound 1
[0172] ((8-((2,2'-Dimethyl-3'-(3-morpholinopropoxy)-[1,1'-biphenyl]-3-yl)amino)-1,7- Naphthyridin-3-yl)methyl)glycine
[0173]
[0174] Step 1: Preparation of 8-chloro-3-vinyl-1,7-naphthyridine (M1)
[0175]
[0176] Toluene (30 mL), EtOH (10 mL), 10% Na 2 CO 3 solution (10 mL) was added Pd(dppf)Cl 2 . DCM (420 mg). 4,4,5,5-Tetramethyl-2-vinyl-1,3,2-dioxaborolane (3.1 g) was added dropwise under nitrogen protection. The mixture was stirred at 100°C for 16 hours. The reaction was quenched with water (50 mL) and extracted 3 times with EtOAc. The organic phases were combined and washed with brine. The resulting solution was concentrated and purified on a silica gel column (gradient from 8:1 to 5:1 with hexane-EtOAc) to afford 8-chloro-3-vinyl-1,7-naphthyridine (1.1 g) 88%).
[0177] Step 2: Preparation of 8-chloro-1,7-naphthyridine-3-carbaldehyde (M2)
[0178]
[0179] To a solution of 8-chloro-3-vinyl-1,7-naphth...
Embodiment 2
[0198] The synthesis of embodiment 2 compound 2
[0199] ((8-((2-Methyl-[1,1'-biphenyl]-3-yl)amino)-1,7-naphthyridin-3-yl)methyl)glycine
[0200]
[0201] Step 1: Preparation of ((8-((2-methyl-[1,1'-biphenyl]-3-yl)amino)-1,7-naphthyridin-3-yl)methyl)glycine methyl ester
[0202]
[0203] This compound was prepared using a method similar to that described for M5 in Example 1, substituting phenylboronic acid for M7. The obtained compound was purified by preparative-TLC (EtOC:n-hexane=1:1) to obtain ((8-((2-methyl-[1,1'-biphenyl]-3-yl]amino]-1,7- Naphthyridin-3-yl)methyl)glycine methyl ester (150 mg) was a yellow solid.
[0204] Step 2: ((8-((2-Methyl-[1,1'-biphenyl]-3-yl)amino)-1,7-naphthyridin-3-yl)methyl)glycine (Compound 3)
[0205]
[0206] This compound was prepared using a method similar to that described in Compound 1. The resulting compound was purified by RP column (mobile phase: MeCN:water (0.1% HCl) with a gradient of 40:60 to 50:50) to give ((8-((2-methy...
Embodiment 3
[0209] The synthesis of embodiment 3 compound 5
[0210] 1-((8-((2,2'-dimethyl-3'-(3-morpholinopropoxy)-[1,1'-biphenyl]-3-yl)amino)-1, 7-naphthyridin-3-yl)methyl)piperidine-2-acetic acid
[0211]
[0212] Step 1: Preparation of (8-chloro-1,7-naphthyridin-3-yl)methanol (M11)
[0213]
[0214] The above aldehyde (620 mg) was dissolved in MeOH. Add NaBH at one time 4 (400mg). The mixture was stirred at room temperature for 2 hours and then quenched with water (30 mL). The mixture was extracted 3 times with DCM (20 mL) and the organic phase was passed through Na 2 SO 4 dry. The resulting solution was concentrated and purified by silica gel column (n-Hexane-EtOAc gradient eluted from 2:1 to 1:1) to give (8-chloro-1,7-naphthyridin-3-yl)methanol (500 mg) as brown solid.
[0215] Step 2: Preparation of (8-((3-bromo-2-methylphenyl)amino)-1,7-naphthyridin-3-yl)methanol (M12)
[0216]
[0217] To a microwave reaction vial was added 3-bromo-2-methylaniline (370 mg), (8-ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com