Particulate composition and production method therefor
A manufacturing method and composition technology, applied in the field of granular composition and its manufacturing, capable of solving undiscovered problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
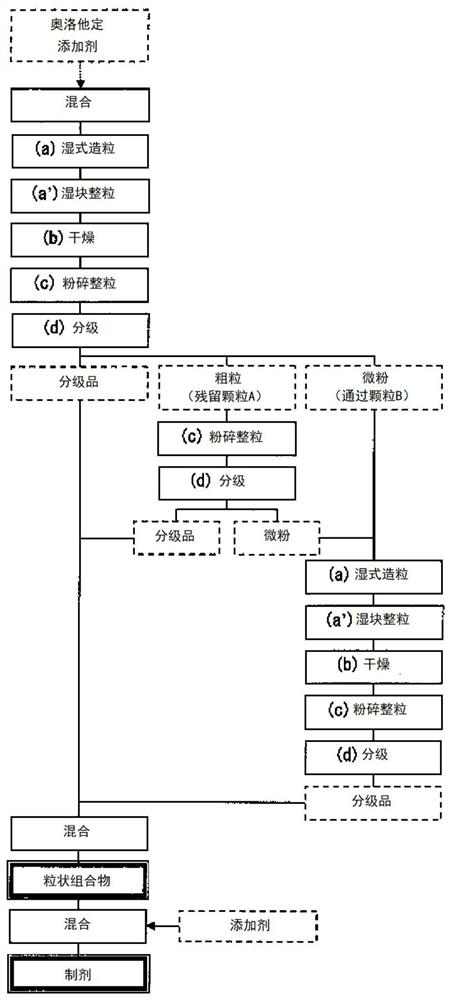
Image
Examples
Embodiment 1
[0130] Example 1: Preparation of Granular Compositions and Formulations
[0131] 64.816 parts of white sugar, 0.4 parts of DL-malic acid, 0.4 parts of dibutyl hydroxytoluene, 24 parts of trehalose hydrate, 1 part of olopatadine hydrochloride, 5 parts of acesulfame potassium, 2 parts of dextrin, light 0.25 parts of quality anhydrous silicic acid, 0.44 parts of edetate sodium hydrate, and 1.06 parts of hydroxypropyl cellulose (HPC) were put into the stirring granulator. A granulation solution prepared by separately dissolving 0.44 parts of HPC and 0.004 part of thaumatin in 5 parts of purified water was put thereinto, and granulated. After the granulation, wet mass sizing was carried out with a crushing and sizing machine, and drying was carried out at 45° C. to obtain a granulated dry product 1 . The granulated dry product 1 was charged into a crushing and sizing machine with a mesh of 1000 μm, and the granules were sized to obtain the sized product 1 . The granulated product...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com