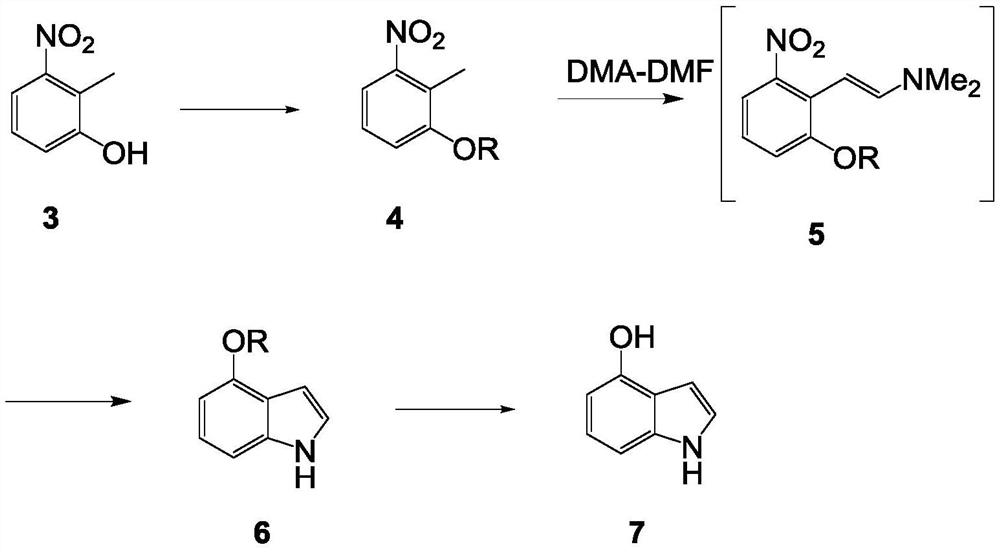
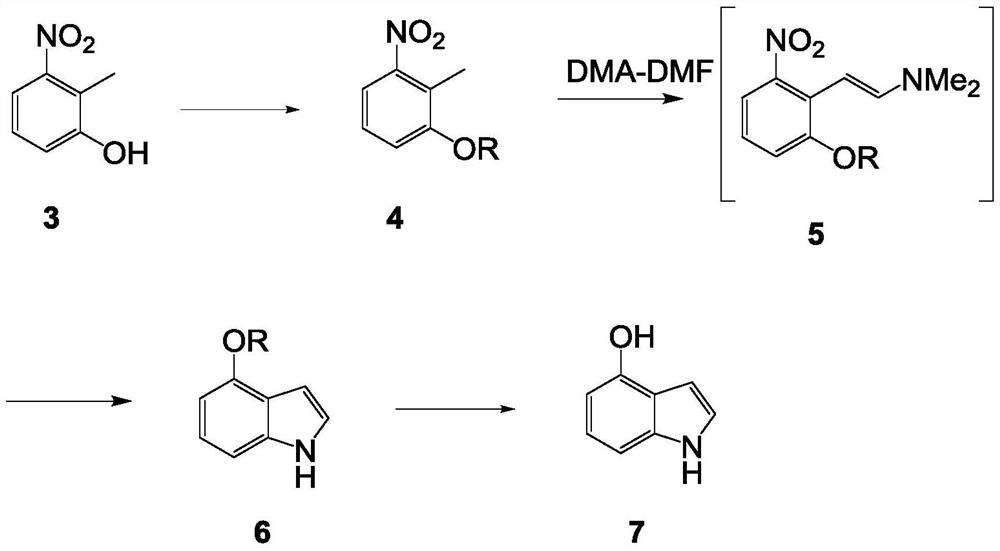
Synthetic method of 4-hydroxyindole
A synthetic method, the technology of oxindole, which is applied in the field of synthesis of 4-oxindole, can solve the problems of high raw material cost, unfavorable industrial production, and harsh reaction conditions of 4-oxindole, and achieve convenient acquisition and reduced production cost , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The synthetic route of present embodiment is as follows:
[0041]
[0042] The method for preparing 4-oxindole in the present embodiment may further comprise the steps:
[0043] 1. Preparation of compound 2:
[0044]
[0045] Specific operation:
[0046](1) 1-chloro-2-methyl-3-nitrobenzene (100g, 1eq), BrettPhos PdG3 (0.53g, 0.001eq), Cs 2 CO 3 (379.78g, 2eq) and methanol (186.74g, 10eq) were added in 500mL toluene, and the reaction solution was 2 Under protection, the temperature was raised to 70°C for 16 hours.
[0047] (2) The remaining control raw material in the TLC plate is less than 5%, and a main point is generated.
[0048] (3) The reaction solution was lowered to room temperature, and added to 1 L of water. The aqueous phase was extracted with ethyl acetate (500 mL×3), and the organic phases were combined. The organic phase was washed with saturated brine (800ML×1), dried over anhydrous sodium sulfate, decolorized by adding 5g of activated carbon,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com