Application of W18O49 modified g-C3N4 material in photocatalytic nitrogen fixation
A technology of W18O49 and g-c3n4, which is applied in the field of photocatalytic nitrogen fixation of g-C3N4 materials, can solve the problems of high production cost, high energy consumption, inability to get rid of organic capture agents or sacrificial agents, etc., and achieve pollution reduction and high nitrogen fixation efficiency , the effect of easy large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
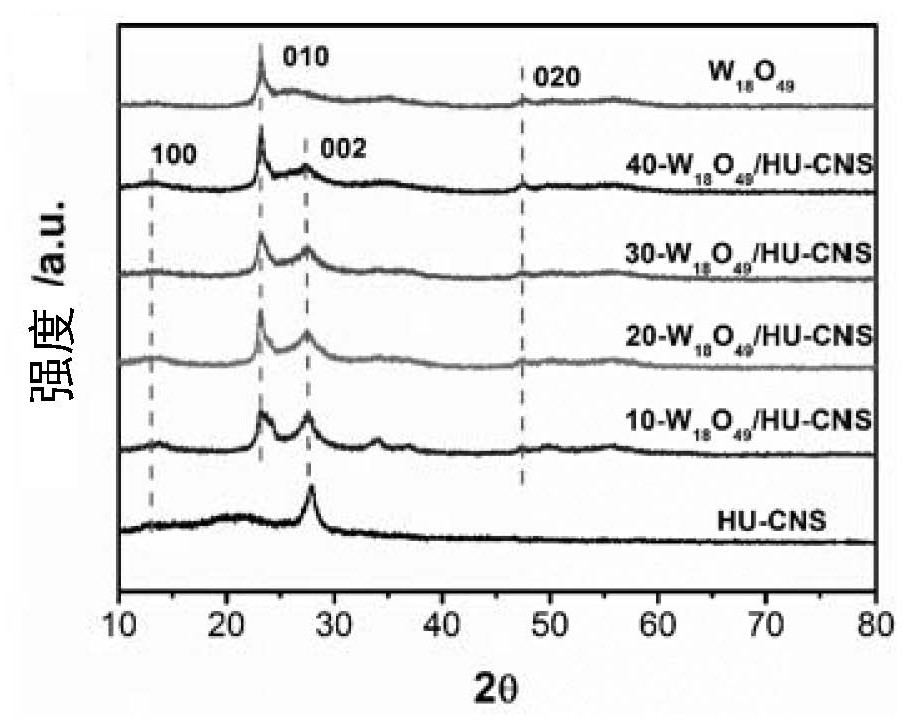
[0029] g-C 3 N 4 Preparation of base material. Melamine is heat-treated at 400-500°C for 1-10 hours, and then ground and pulverized to obtain the g-C 3 N 4 (CNB) as g-C 3 N 4 Matrix material. The heat treatment system includes: first raising the temperature at 1-10°C to 400-500°C and then keeping it warm for 1-10 hours, then raising the temperature at 1-10°C to 500-600°C and keeping it warm for 1-10 hours. Or further, put g-C 3 N 4 Heat treatment in air at 1-10°C for 1-10 hours to obtain ultra-thin porous g-C 3 N 4 (HU-CNS) as g-C 3 N 4 Matrix material.
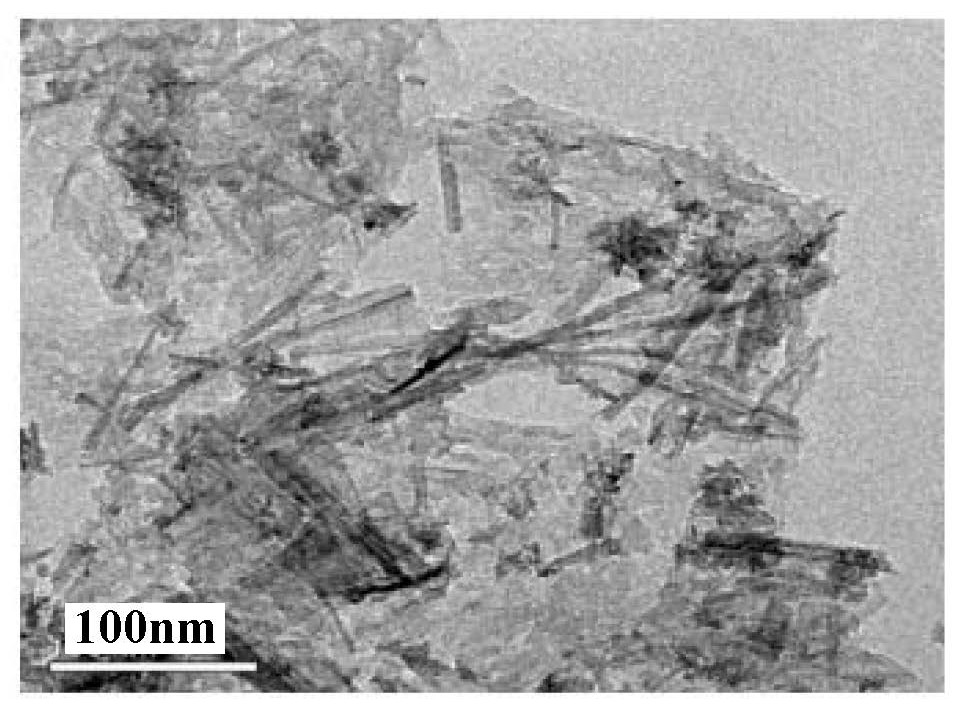
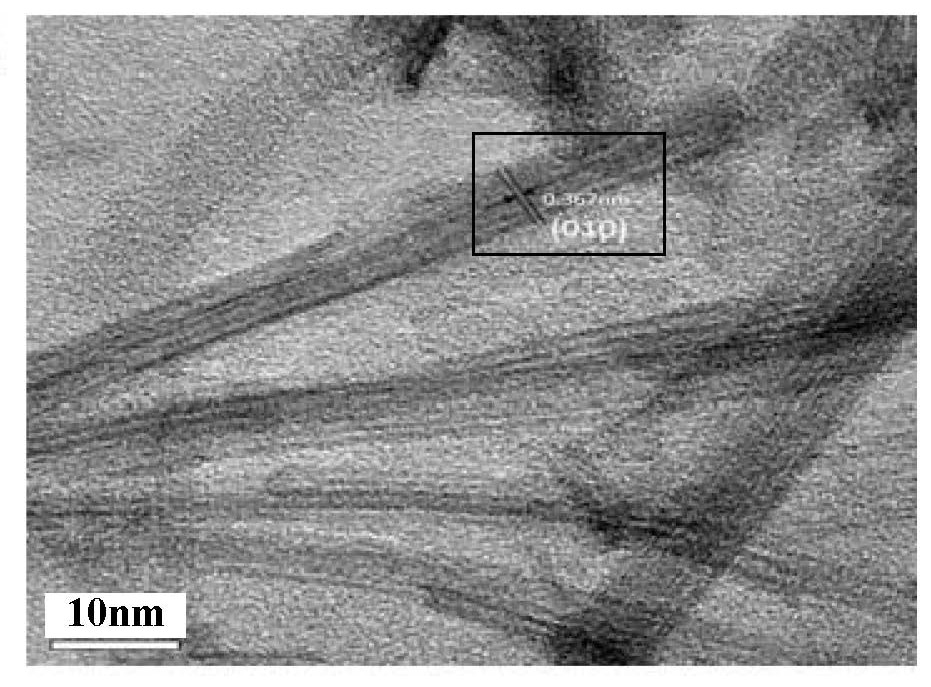
[0030] Add CNB to WCl 6 In the ethanol solution, hydrothermally react at 100-200°C for 12-36 hours, and grow superfine W on its surface hydrothermally 18 o 49 nanowire, get W 18 o 49 / CNB composite catalyst. The WCl 6 The quality can be 1 ~ 10g. The volume of ethanol can be 10~100ml. The g-C 3 N 4 The mass can be 0.1 ~ 1g.
[0031] ultrathin porous g-C 3 N 4 Added to WCl 6 In the ethanol solution, h...
Embodiment 1
[0044] Embodiment 1: Modification of different proportions of W 18 o 49 Photocatalytic nitrogen fixation experiment of HU-CNS material in water phase:
[0045] (1) Weigh a certain amount of melamine (5g) and place it in an alumina crucible, then transfer it to a muffle furnace and raise the temperature to 500°C for 2 hours at a heating rate of 2°C / min, and then heat it at a heating rate of 10°C / min Raise the temperature to 520°C for 2 hours, cool and wash to obtain the bulk phase g-C 3 N 4 (CNB);
[0046] (2) Weigh a certain amount of bulk CNB (1.5g) and grind it, evenly disperse it on the alumina crucible cover, place it in a muffle furnace and raise the temperature to 520°C at a heating rate of 2°C / min for 6 hours, then cool Wash to get ultra-thin porous g-C 3 N 4 (HU-CNS);
[0047] (3) A certain amount of WCl 6 (0.1g) was dissolved in ethanol (10ml) solution, stirred, and dispersed evenly by ultrasonic; respectively weighed HU-CNS (0g, 0.513g, 0.228g, 0.133g, 0.086g...
Embodiment 2
[0053] Example 2: W 18 o 49 / HU-CNS with optimal ratio of 30-W 18 o 49 / HU-CNS continuous nitrogen fixation experiment
[0054] (1) Prepare 30-W 18 o 49 / HU-CNS composite material (with embodiment 1);
[0055] (2) will get 30-W 18 o 49 After the / HU-CNS composite material is fully ground, take 0.05g of powder and add it to 200mL of water, put it in a 600mL reactor, the reactor is an open system, and stir for 0.5h in the dark to conduct the adsorption-desorption equilibrium experiment;
[0056] (3) Turn on the xenon lamp and illuminate for 1 hour under the simulated sunlight light source;
[0057] (4) Suction filtration, take 50mL of the filtrate and put it in a colorimetric tube, add 1mL potassium sodium tartrate and 1mL Nessler's reagent, shake well, let it stand for 10-15min, and measure the ammonia production with a UV spectrophotometer when the color develops stably.
[0058] The yield of ammonia obtained in the experiment is as image 3 shown, 30-W 18 o 49 The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com