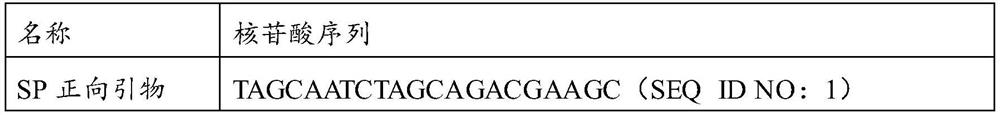Kit for simultaneously detecting streptococcus pneumoniae, legionella pneumophila and Moraxella catarrhalis
A technology for Streptococcus pneumoniae and Legionella pneumophila, applied in the field of fluorescent PCR detection, can solve the problems of long time, easy missed diagnosis, high nutritional requirements, and achieve the effect of ensuring timeliness, fast detection method, and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
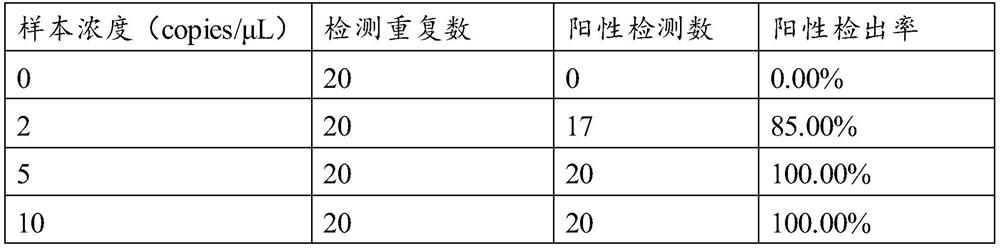
[0049] 3. Preparation of quality control products
[0050] Positive quality control: Mix cultures of Streptococcus pneumoniae, Legionella pneumophila and Moraxella catarrhalis 1:1:1; dilute 1000 times, aliquot 0.65mL. Negative quality control: β-globin pseudovirus diluted and mixed with virus preservation solution, aliquoted into 0.65mL.
Embodiment 1
[0052] Embodiment 1 simultaneously detects the preparation of the kit of Streptococcus pneumoniae, Legionella pneumophila and Moraxella catarrhalis
[0053] The kit includes PCR reaction solution, negative quality control, and positive quality control;
[0054] Wherein, the components of the PCR reaction solution are shown in Table 2:
[0055] Table 2 The components and concentrations in the PCR reaction solution
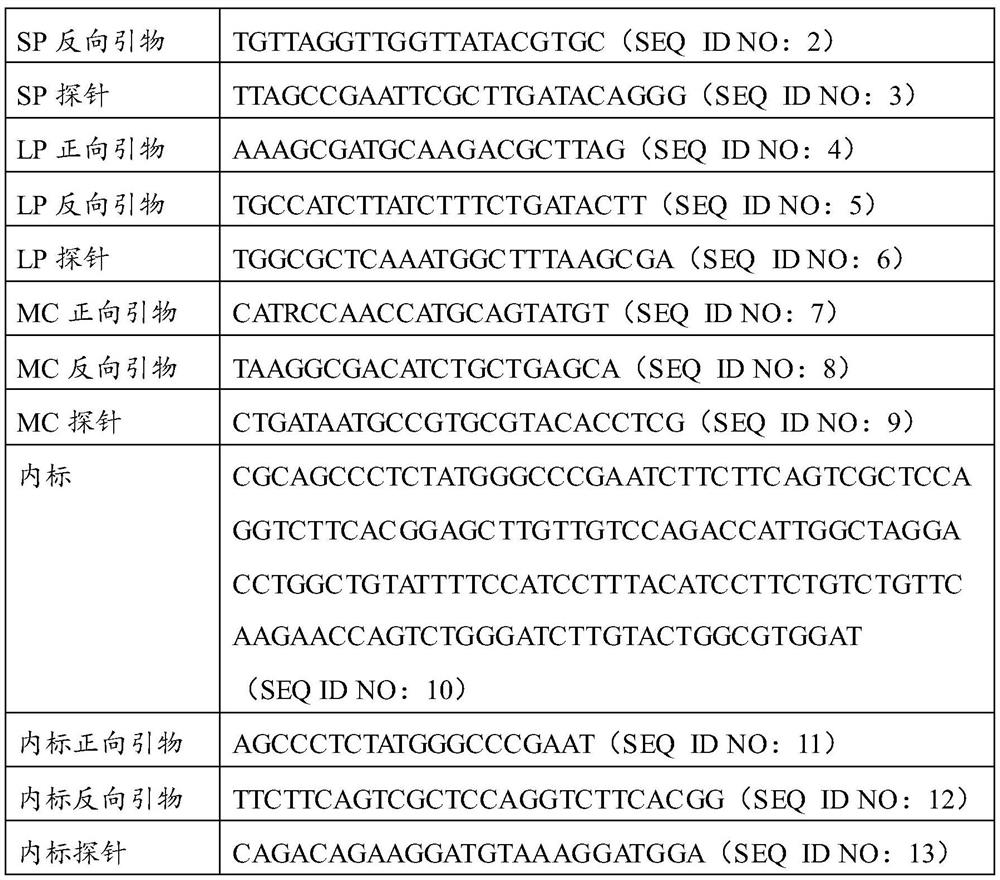
[0056] components volume in each reaction system reaction buffer 10 μL dNTPs (10mM) 2μL MLV enzyme (200U / μL) 0.1μL Taq enzyme (5U / μL) 0.3μL MgCl 2 (50mM)
2μL SEQ ID NO: 1 1μL SEQ ID NO: 2 1μL SEQ ID NO: 3 0.5μL SEQ ID NO: 4 1μL SEQ ID NO: 5 1μL SEQ ID NO: 6 0.5μL SEQ ID NO: 7 1μL SEQ ID NO: 8 1μL SEQ ID NO: 9 0.5μL SEQ ID NO: 11 0.5μL SEQ ID NO: 12 0.5μL SEQ ID NO: 13 0.25 μL Sterilized purified water Up To 25μL
[0057] P...
Embodiment 2
[0059] The detection method of embodiment 2 kit of the present invention
[0060] Mix the human sputum sample with a pipette, take out 200 μL of sputum, add 4 times the volume of 4% NaOH (self-prepared), shake well, and leave it at room temperature for 30 minutes to liquefy (if the sputum is still very viscous, you can increase the storage time Until the sputum is liquefied), take 0.5mL sample to a 1.5mL centrifuge tube, then add 0.5mL 4% NaOH and leave it at room temperature for 10 minutes, then centrifuge at 13000rpm for 5 minutes. Add 1 mL of sterile saline to the precipitate, mix well, and centrifuge at 13,000 rpm for 5 minutes; repeat the washing once more, and remove the supernatant. Add 200 μL of bacterial lysate (from Beijing Biolab Technology Co., Ltd.) to the precipitate, mix well, bathe in water at 100°C for 10 minutes, and centrifuge at 13,000 rpm for 5 minutes for later use.
[0061] Streptococcus pneumoniae, Legionella pneumophila and Moraxella catarrhalis nucle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com