Synthetic method of polypeptide hydrazide containing cysteine residues and application of synthetic method
A technology of cysteine residues and polypeptide hydrazides, which is applied in the field of polypeptide/protein synthesis and preparation, can solve problems such as incomplete reactions, large site restrictions, and decreased solubility of polypeptides, so as to improve yield and efficiency, and broaden the scope of production. Range of application, effect of improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072]Example 1: Synthesis of ASHC4 Simulated Peptide Polypeptide
[0073](1) dissolve polypeptide and reduce treatment
[0074]The synthetic ASHC4-CCPGCC-AKA is dissolved in 6 mol / l GDMCl, 0.2mol / L Na2HPO41 mmol / LEDTA, pH 7.4 buffer formulated with 1 mmol / L of polypeptide solution, TCEP having a final concentration of 10 mmol / L was added, and the penetration reaction was 30 min under 37 ° C to ensure that diopene is not formed between the polypeptide. key. The reverse HPLC was monitored, and the method was completed in accordance with whether or not only the target molecular peak is determined in 20 minutes from 25% in 20 minutes to 45% in 20 minutes.
[0075](2) Polypeptide and Flash-EDT2Coupled reaction
[0076]Take the above solution, add Flash-EDT soluble in DMSO2The final concentration of the peptide was 1 mmol / L, stirred at room temperature, stirring at room temperature, and the reaction was stirred at room temperature for 30 min, and the reaction was monitored with MALDI-TOF...
Embodiment 2
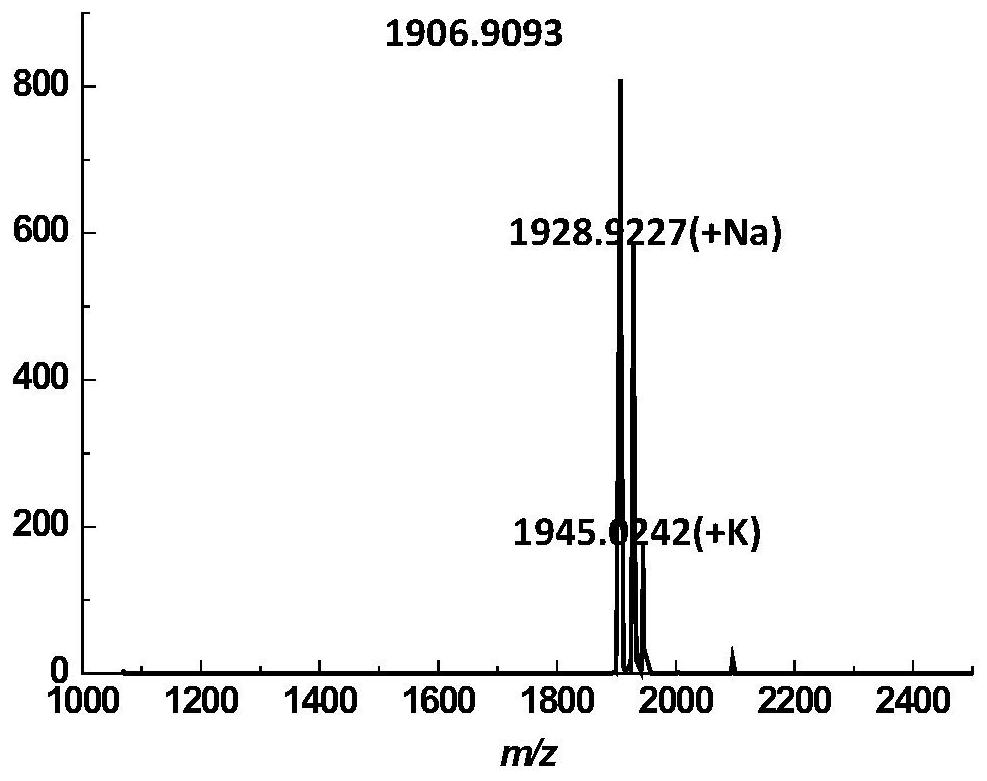
[0092]Example 2: Synthesis of Rubredoxin F1 Polypeptide
[0093]Referring to the method of Example 1, it was prepared to obtain Rubredoxin F1 polypeptide acyl hydrazine.Figure 8A Maldi-TOF for the product with a yield of 48%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com