A kind of Lycium barbarum ace, dpp-iv inhibitory peptide and derivative polypeptide and application, mixture
A technology of DPP-IV and inhibitory peptides, applied in application, peptide, drug combination, etc., can solve the problems of liver and kidney tissue burden, toxic side effects, liver and kidney damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
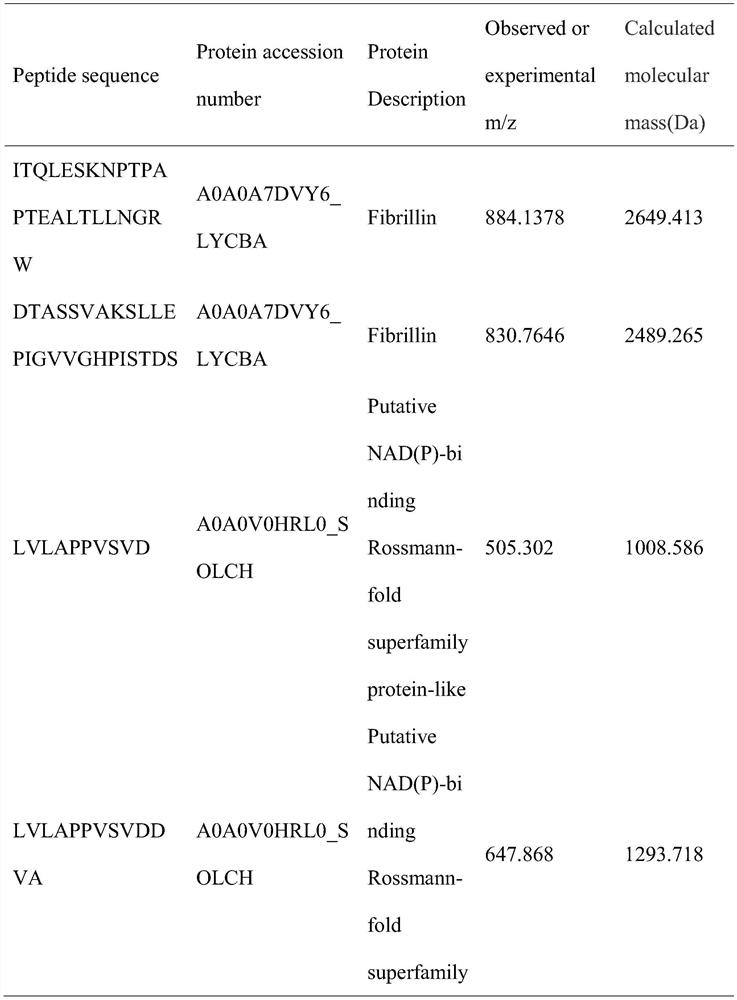
[0016] The identification of embodiment 1 polypeptide LLEPIGVVGH
[0017] Lycium barbarum protein was subjected to Bacillus subtilis fermentation, acid precipitation and ethanol precipitation, and LC-MS / MS analysis, and bioactive peptides with inhibitory activity on ACE were screened by bioinformatics and structure-activity relationship.
[0018] The specific method is as follows:
[0019] (1) Preparation of wolfberry protein extract
[0020] Using 10g of dried wolfberry as raw material, weigh wolfberry and add 100mL of deionized water to soak for 2 hours, then pulverize, ultrasonically extract for 60min, centrifuge at 5000rpm for 10min, and 9.6g of precipitate is used to extract wolfberry protein.
[0021] After precipitation and freeze-drying, pulverize again to obtain wolfberry powder, add 192mL of organic solvent extract to the wolfberry powder, n-hexane:ethanol=2.6:1 (v / v), stir and extract at a temperature of 50°C and a rotating speed of 150rpm for 1 hour, filter, filte...
Embodiment 2
[0030] Example 2 Detection of ACE inhibitory activity of Lycium barbarum active peptide LLEPIGVVGH
[0031] Polypeptide LLEPIGVVGH was synthesized by Nanjing Jiepeptide Biotechnology Co., Ltd., the amino acid sequence is Leu-Leu-Glu-Pro-Ile-Gly-Val-Val-Gly-His, it is a single-chain linear structure, white powder, soluble in water, The molecular weight is 1033.24Da.
[0032] Information on SEQ ID No.1
[0033] (a) Sequential features
[0034] *Length: 10 amino acids
[0035] *Type: amino acid
[0036] * Chain type: single chain
[0037] (b) Molecule type: protein
[0038] Sequence description:
[0039] SEQ ID No.1
[0040] LLEPIGVVGH
[0041] N-(3-(2-Furoyl)acryloyl-phenyl-glutamyl-glutamic acid (FAPGG, λmax=340nm, ε=2270M -1 cm -1 , molecular weight 399.40) can be enzymatically hydrolyzed by ACE into N-[3-(furyl)acryloyl]-2-phenylalanine (FAP, λmax=340nm, ε=1512M -1 cm -1 ) and glycylglycyl peptide (GG, no absorption at 340nm), so it can be used as the mimic substr...
Embodiment 3
[0059] Example 3 Detection of DPP-IV inhibitory activity of Lycium barbarum active peptide LLEPIGVVGH
[0060] DPP-IV can degrade glucagon-like peptide-1 (GLP-1) because GLP-1 has an X-Ala structure. For peptides with N-terminal X-Pro and X-Ala, DPP-IV The dipeptide can be selectively cleaved. In this experiment, we chose Gly-Pro-p-nitroanilide instead of GLP-1 as the simulated substrate of DPP-IV. The generated p-nitroaniline has absorption at 405nm, and the inhibitory activity of DPP-IV can be calculated by absorbance.
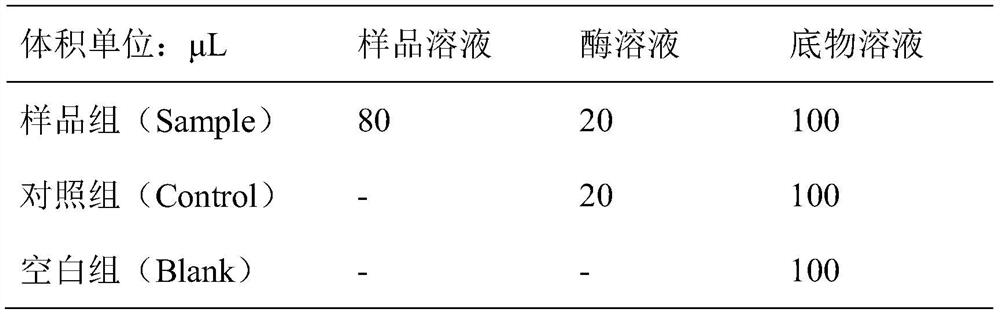
[0061] The reaction system is:
[0062] (1) Buffer solution: 100 mM Tris-HCl buffer (pH=8.0).
[0063] (2) Substrate solution: Gly-pro-p-nitroanilide solution with a concentration of 1.59 mM was prepared using the above buffer solution.
[0064] (3) Enzyme solution: use the above buffer solution to prepare 0.01 U / mL DPP-IV solution.
[0065] (4) Sample solution: The polypeptide LLEPIGVVGH was prepared with the above-mentioned buffer solution into solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com