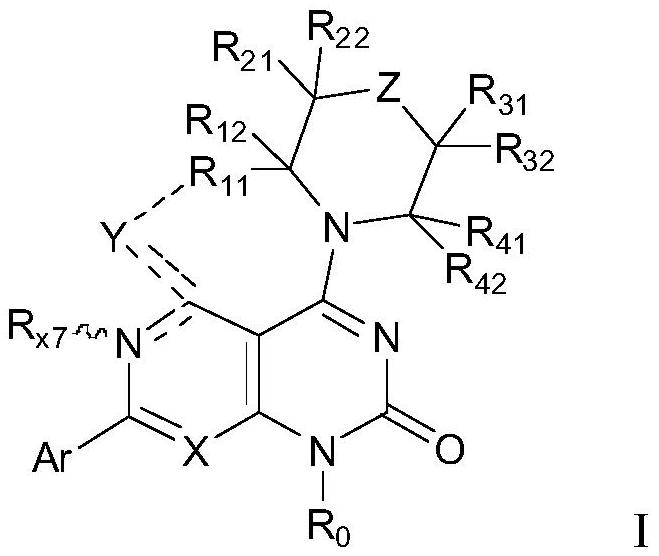
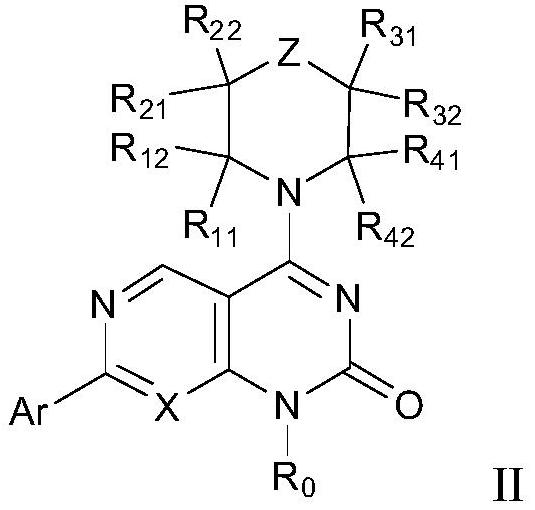
Substituted heteroaromatic ring dihydropyrimidinone derivative as well as preparation method and medical application thereof
A pharmacy and prodrug technology, applied in the field of medicine, can solve problems such as cancer, prolong cell signal transduction, prolong protein activation time, etc., and achieve the effects of low toxicity and side effects, good selectivity and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0171] Example 1 Preparation of (S)-7-acryloyl-2-(2-fluoro-6-hydroxyphenyl)-12-(2-isopropyl-4-methylpyridin-3-yl)-5a,6 ,7,8,9-Hexahydro-4-oxa-3,7,9a,10,12-pentaazabenzo[4,5]cyclohepta[1,2,3-de]-naphthalene- 11(12H)-Kone (Z1)
[0172]
[0173] Step 1: Dissolve 2,4,6-trichloronicotinic acid (8g, 35.5mmoL) in 150mL of dichloromethane, cool down to 0°C, add oxalyl chloride (9.3ml, 106.6mmol), drop after 30 minutes Add DMF (0.5ml), rise to room temperature and react for 1h. After the reaction is completed, concentrate and dilute with 150mL of dichloromethane, lower to 0°C, slowly add 8mL of ammonia water dropwise, stir at room temperature for 2h, after the reaction is completed, concentrate, and dilute with ethyl acetate Slurry and filter to obtain 6g (6.5g, yield: 82%) of crude product 2,4,6-trichloronicotinamide. ES-API:[M+H] + = 224.9.
[0174] Step 2: Dissolve 2-isopropyl-4-methylpyridin-3-amine (4.4g, 29.1mmol) in 80mL tetrahydrofuran, under nitrogen protection, add LiH...
Embodiment 2
[0183] Example 2 Preparation of (S)-7-acryloyl-12-(2-isopropyl-4-methylpyridin-3-yl)-2-(5-methyl-1H-indazol-4-yl) -5a,6,7,8,9-hexahydro-4-oxa-3,7,9a,10,12-pentaazabenzo[4,5]cyclohepta[1,2,3-de ]naphthalene-11-(12H)-one (Z2)
[0184]
[0185] Step 1: Add (S)-2-chloro-12-(2-isopropyl-4-methylpyridin-3-yl)-11-oxo-5a,6,8,9,11,12-hexa Hydrogen-4-oxa-3,7,9a,10,12-pentaazabenzo[4,5]cyclohepta[1,2,3-de]-naphthalene-7(5H)-carboxylic acid tertiary Butyl ester (130mg, 0.247mmoL), (5-methyl-1H-indazol-4-yl) boronic acid (86.8mg, 0.493mmol), Pd(PPh 3 ) 4 (28.5mg, 0.0247mmol) and potassium carbonate (68g, 0.493mmol) were dissolved in 2ml dioxane and 0.2ml water, replaced with nitrogen, reacted at 120°C for 1.5h, cooled to room temperature, filtered, passed through water and saturated saline Washing, concentration, and column chromatography to obtain (S)-12-(2-isopropyl-4-methylpyridin-3-yl)-2-(5-methyl-1H-indazol-4-yl )-11-oxo-5a,6,8,9,11,12-hexahydro-4-oxa-3,7,9a,10,12-pentaazabenz...
Embodiment
[0189] Compounds Z3, Z5 to Z10 and Z15 were prepared with reference to the similar methods of compound Z1 or Z2, wherein the starting materials of each compound can be prepared by commercially available or with reference to existing methods well known to those skilled in the art, and the similar synthetic methods of intermediates It is easily obtained by those skilled in the art by referring to existing methods.
[0190]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com