Equivalent signal mining method for adverse reaction signals of srs combined medication
A technology for adverse reactions and combined medication, which is applied in the fields of medical data mining, informatics, and drug reference. It can solve the problems of complex drug combination mode, large number of signal clues, and few positive signals, and avoid SRS data query. , the effect of improving computing efficiency and reducing workload
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0051] The specific implementation manners of the present invention will be further described in detail below in conjunction with the accompanying drawings and embodiments. The following examples are used to illustrate the present invention, but are not intended to limit the scope of the present invention.
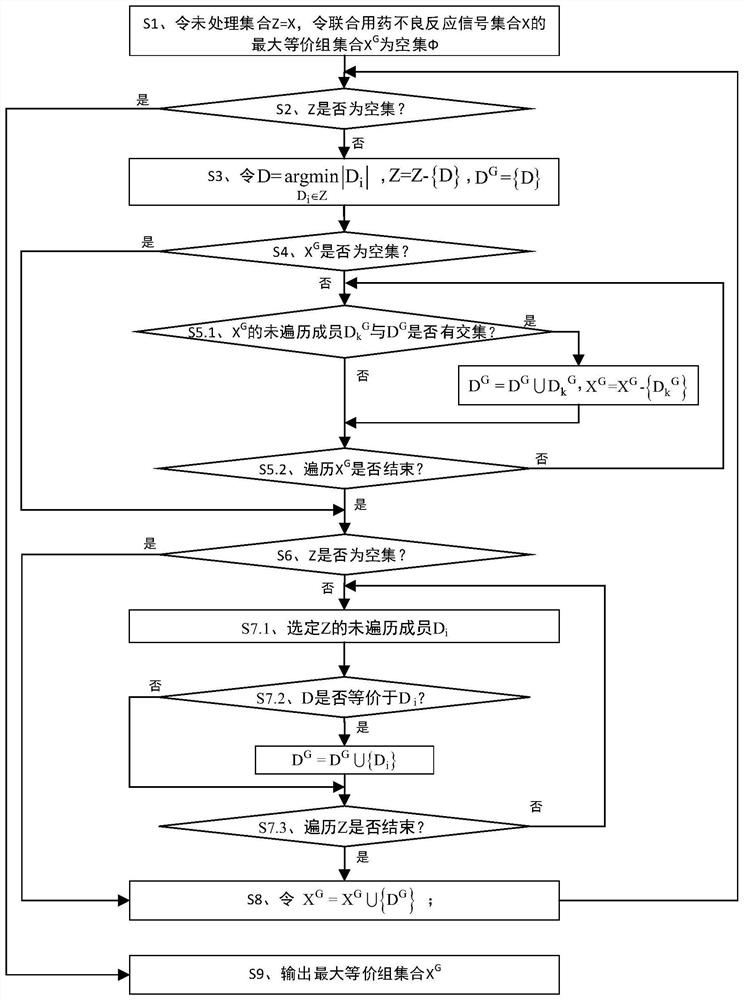
[0052] The present invention uses the adverse event report file issued by FAERS to analyze the mining method of the adverse reaction equivalence relationship signal of combined drug bleeding, such as figure 1 shown, including:
[0053] Phase I acquires data through SRS and preprocesses it: acquires data through SRS, deduplicates data, standardizes drug names, and selects target AEs. This is the basis and premise of all signal mining work. After data preprocessing, it is used for signal analysis in the last two stages. For the convenience of expression below, the following symbols are defined: Let all patient sets in SRS be recorded as PS={p 1 ,p 2 ,...,p m}, all drug...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com