Method for manufacturing diisocyanate and optical lens
A kind of technology of diisocyanate and m-xylylene diisocyanate, applied in the field of preparing diisocyanate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
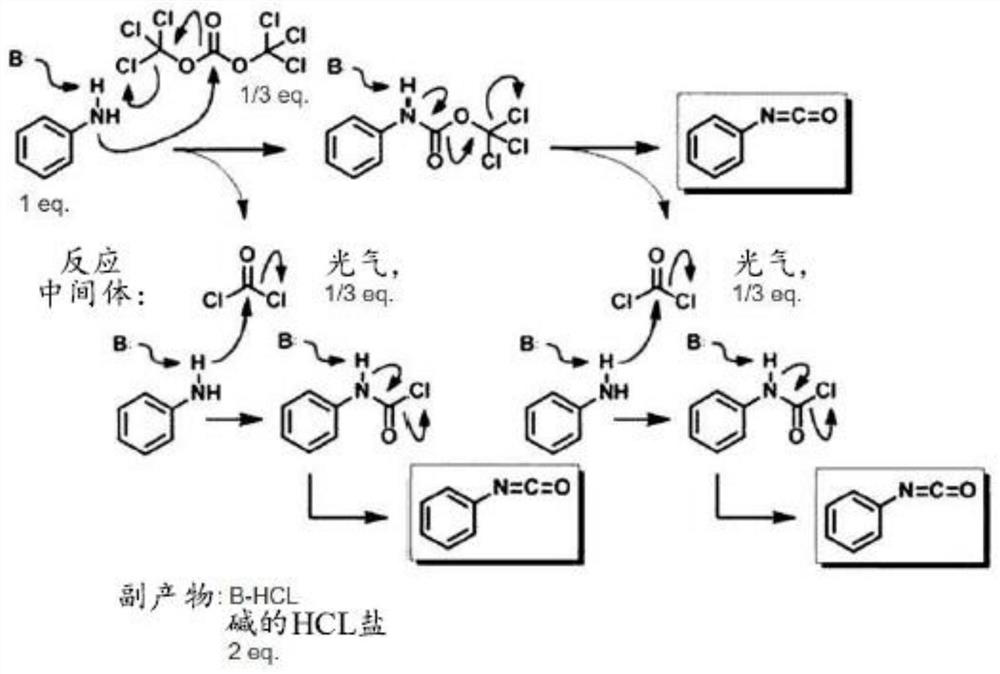
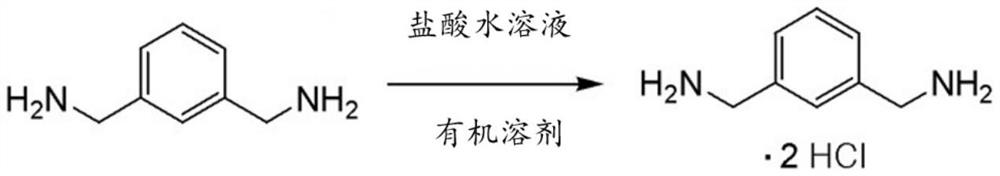
[0033] A method for preparing a diisocyanate according to an embodiment includes preparing at least one diamine selected from the group consisting of o-xylylenediamine, m-xylylenediamine, p-xylylenediamine, norbornenediamine, hydrogenated benzene Dimethylamine, and isophorone diamine; making said diamine react with aqueous hydrochloric acid in a first solvent to obtain diamine hydrochloride; and making said diamine hydrochloride and triphosgene react in a second Reaction in organic solvents to obtain diisocyanates.
[0034]In the method for preparing diisocyanate according to the embodiment, the diamine used as a raw material may be at least one selected from the group consisting of o-xylylenediamine (o-XDA), m-xylylenediamine (m- XDA), p-xylylenediamine (p-XDA), norbornenediamine (NBDA), hydrogenated xylylenediamine (H6XDA), and isophoronediamine (IPDA).
[0035] Preparation of diamine hydrochloride
[0036] First, diamine is reacted with aqueous hydrochloric acid in a firs...
specific Embodiment approach
[0177] Hereinafter, more specific embodiments are shown, but the present invention is not limited thereto.
[0178]
example 1
[0180] Into a 5-liter 4-neck reactor was charged 963.5 g (9.25 mol) of 35% aqueous hydrochloric acid, and then the internal temperature of the reactor was lowered to 15°C to 20°C while stirring. While maintaining the temperature of the reactor at 25°C to 50°C, 600.0 g (4.4 moles) of m-xylylenediamine (m-XDA) was introduced for 1 hour. After the introduction was completed, the internal temperature of the reactor was lowered to 10°C to 20°C, and it was stirred for 1 hour. Thereafter, 1,200.0 g of diethyl ether (Et 2 O) as an organic solvent, and lower the internal temperature of the reactor to -5°C to 0°C, and then stir for 30 minutes to 1 hour. After the reaction was completed, it was vacuum filtered using a filter, and the filtered diethyl ether was recovered for reuse. The recovery of diethyl ether was 73%. After vacuum filtration, m-xylylenediamine (m-XDA) hydrochloride was obtained. In order to remove the residual organic solvent and water, drying was carried out under ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| transmittivity | aaaaa | aaaaa |
| haze | aaaaa | aaaaa |
| haze | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com