Method for reducing content of miscellaneous lithium in high-nickel positive electrode material
A cathode material, high nickel technology, applied in the field of lithium ion batteries, can solve the problems of destroying the lattice stability of high nickel materials and high cost, improving the decrease of electron and lithium ion diffusion rate, improving lattice stability, and saving costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
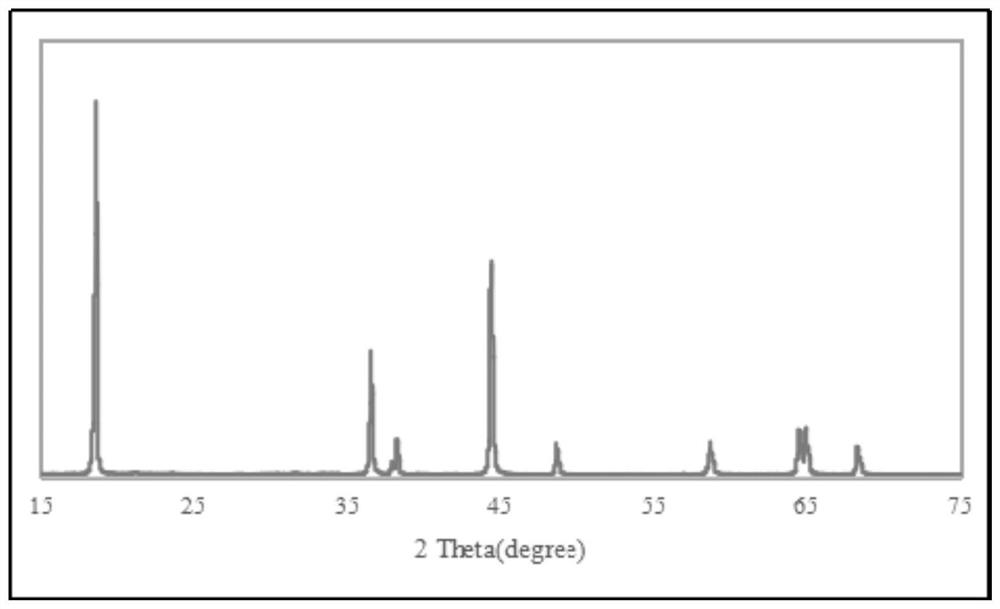
Image
Examples
Embodiment 1
[0031] With 1000g nickel-cobalt-aluminum molar ratio 88:7:5 particle diameter D50 is the nickel-cobalt-aluminum composite hydroxide precursor of 18um, and 1.81g Mg(OH) 2 And 473g lithium hydroxide monohydrate (molar ratio Li:Me=1.025:1) joins in the small-scale high-speed mixer, unloads after mixing 0.5h, packs in the sagger, burns in oxygen atmosphere 780 ℃ (overburning (calcination temperature 50°C) for 10h, then 500g of one-fired NCA and 2.86g of boron oxide were fired at 250°C for 6h to obtain a high-nickel cathode material A.
[0032] With 1000g nickel-cobalt-aluminum molar ratio 88:7:5 particle diameter D50 is the nickel-cobalt-aluminum composite hydroxide precursor of 2.3um, and 1.81g Mg(OH) 2 And 473g lithium hydroxide monohydrate (molar ratio Li:Me=1.025:1) joins in the small-scale high-speed mixer, unloads after mixing 0.5h, packs in the sagger, burns (overburned at 740 ℃ in oxygen atmosphere) (calcination temperature 40°C) for 10h, and then 500g of one-fired NCA an...
Embodiment 2
[0036] With 1000g nickel-cobalt-aluminum molar ratio 88:7:5 particle diameter D50 is the nickel-cobalt-aluminum composite hydroxide precursor of 18um, and 1.81g Mg(OH) 2 And 473g lithium hydroxide monohydrate (molar ratio Li:Me=1.025:1) joins in the small-scale high-speed mixer, unloads after mixing 0.5h, packs in the sagger, burns in oxygen atmosphere 810 ℃ (overburning (calcination temperature 80°C) for 10h, and then 500g of one-fired NCA and 2.86g of boron oxide were fired at 250°C for 6h to obtain a high-nickel cathode material.
[0037] With 1000g nickel-cobalt-aluminum molar ratio 88:7:5 particle diameter D50 is the nickel-cobalt-aluminum composite hydroxide precursor of 2.3um, and 1.81g Mg(OH) 2 And 473g lithium hydroxide monohydrate (molar ratio Li:Me=1.025:1) joins in the small-scale high-speed mixer, unloads after mixing 0.5h, packs in the sagger, burns in oxygen atmosphere 780 ℃ (overburning (calcination temperature 80°C) for 10h, and then 500g of one-fired NCA and...
Embodiment 3
[0040] With 1000g nickel-cobalt-aluminum molar ratio 88:7:5 particle diameter D50 is the nickel-cobalt-aluminum composite hydroxide precursor of 18um, and 1.81g Mg(OH) 2 And 473g lithium hydroxide monohydrate (molar ratio Li:Me=1.025:1) joins in the small-sized high-speed mixer, unloads after mixing 0.5h, packs in the sagger, burns (overburned at 760 ℃ in oxygen atmosphere) (calcination temperature 30°C) for 10h, and then 500g of one-fired NCA and 2.86g of boron oxide were fired at 250°C for 6h to obtain a high-nickel cathode material A.
[0041] With 1000g nickel-cobalt-aluminum molar ratio 88:7:5 particle diameter D50 is the nickel-cobalt-aluminum composite hydroxide precursor of 2.3um, and 1.81g Mg(OH) 2 And 473g lithium hydroxide monohydrate (molar ratio Li:Me=1.025:1) joins in the small-sized high-speed mixer, unloads after mixing 0.5h, packs in the sagger, burns in oxygen atmosphere 730 ℃ (overburning (calcination temperature 30°C) for 10h, and then 500g of one-fired NC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com