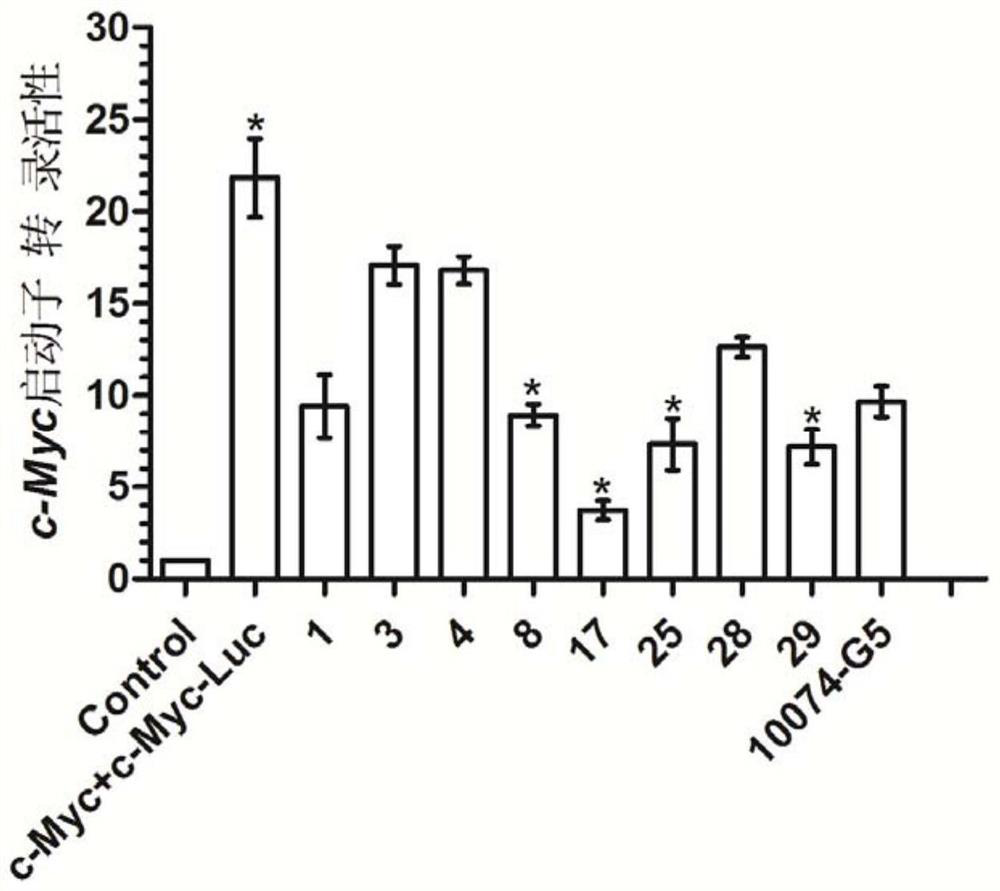
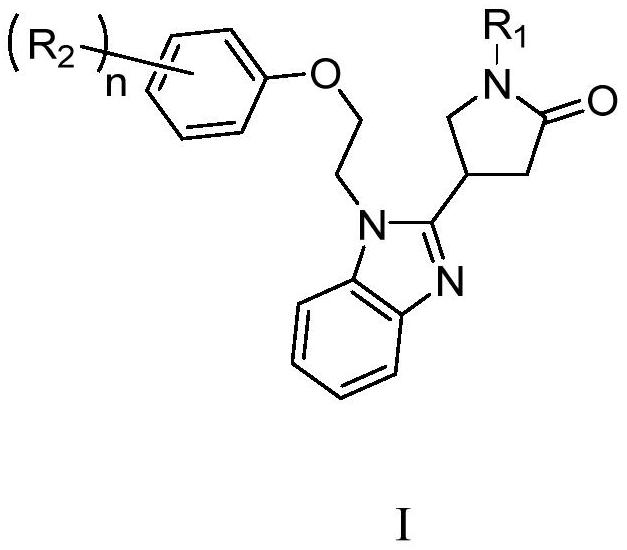
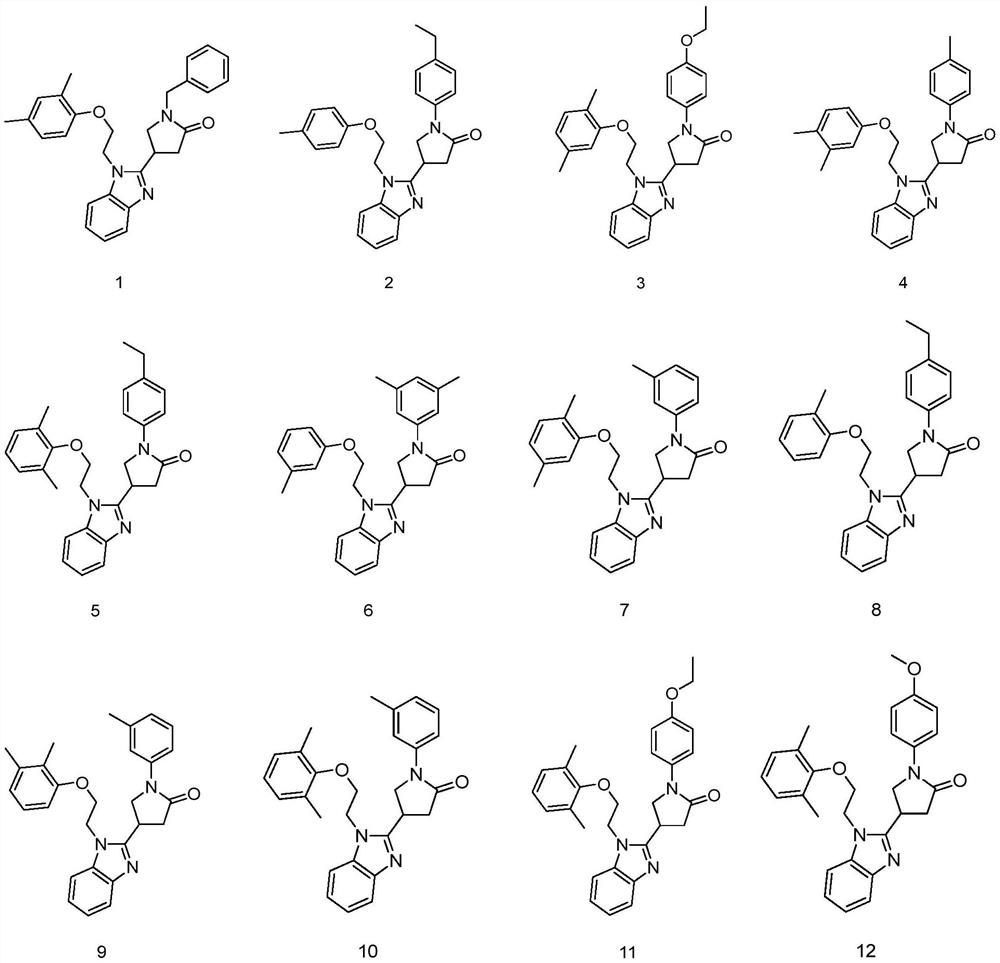
Application of pyrrolidin-2-one compounds in the preparation of drugs related to multiple myeloma
A multiple myeloma, compound technology, applied in the field of medicinal chemistry and pharmacotherapeutics, can solve the problems of undruggability, key interaction site lag, etc., and achieve good c-Myc inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Experimental methods and results
[0081] 1. Cell Viability Experiment
[0082] Experimental principle: The reagent contains WST-8, which is reduced by the dehydrogenase in the cell under the action of the electron carrier 1-methoxy-5-methylphenazinium dimethyl sulfate (1-Methoxy PMS) Highly water-soluble yellow formazan product (Formazan dye). The amount of formazan produced is directly proportional to the number of living cells. This feature can therefore be used directly for cell proliferation and toxicity assays.
[0083] Experimental steps:
[0084] 1. Plating: Two kinds of humanized myeloma cells in the logarithmic growth phase, RPMI-8226 and U266, were inoculated in a 96-well plate, with 100 μL of cell suspension per well, and the number of cells was 5×10 3 For the blank control group, only 100 μL of complete medium RPMI-1640 containing 10% FBS was added, and 3 to 5 duplicate wells were set for each group.
[0085] 2. RPMI-8226 and U266 treated with differen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com