P-dimethylaminostyrylquinoline derivative, synthesis method thereof and application thereof in nucleic acid fluorescent probe
A technology of dimethylaminostyrene quinoline and fluorescent probes, which is applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of low selectivity of nucleic acid probes, high toxicity and light stability, and achieve Effect of low detection limit, strong binding constant, and high fluorescence intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the synthesis of intermediate a
[0043] 4-Chloro-2-methylquinoline (1.5 mmol) and iodomethane (4.5 mmol) were added to a closed pressure reactor, and then the compound was dissolved with sulfolane (6 mL). The mixture was heated and reacted at 60°C for 12 hours. After the reaction, the mixture was cooled to room temperature, and ethyl acetate (15 mL) was added to precipitate the crude product. Recrystallized in ethanol and dried to obtain dark green pure product.
[0044] The reaction process is shown in the following formula:
[0045]
Embodiment 2
[0046] Embodiment 2: the synthesis of compound B1
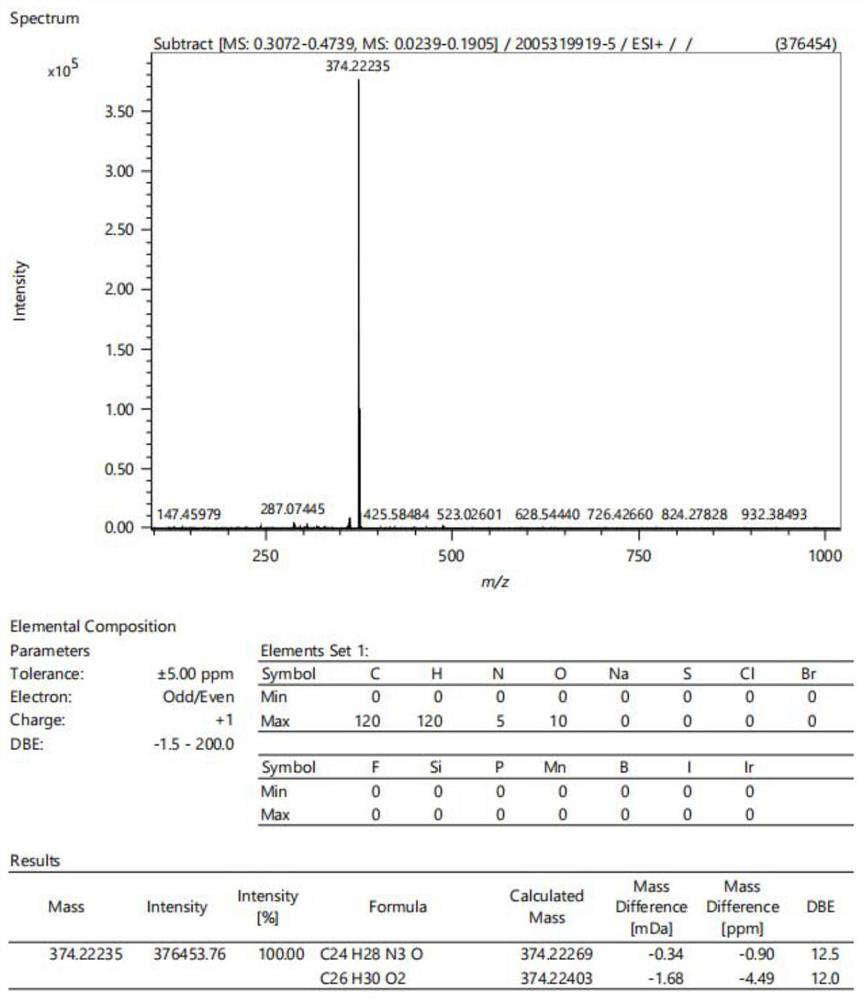
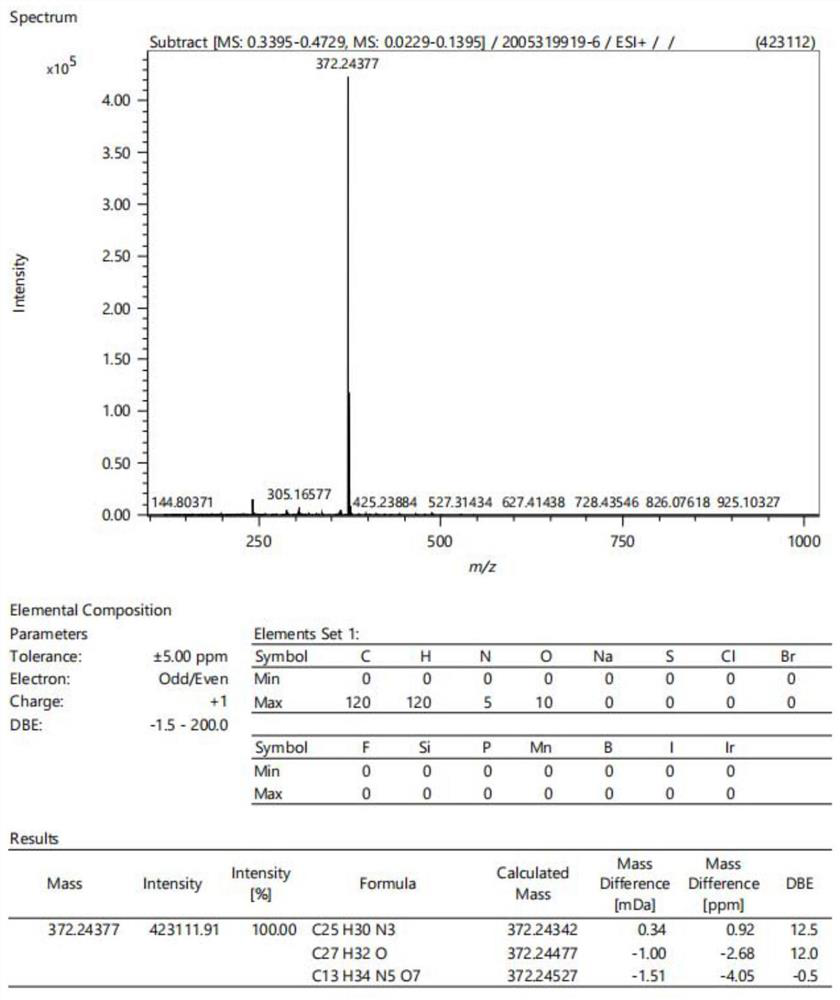
[0047]Intermediate a (0.2 mmol), morpholine (0.6 mmol) and 4-dimethylaminobenzaldehyde (0.4 mmol) were added to the flask. The compound was dissolved with n-butanol (4 mL). The mixture was stirred and refluxed at 80 °C for 8 h. When the reaction was complete, the mixture was cooled to room temperature, then ethyl acetate (10 mL) was added. After standing still for 30 min, the crude product was obtained by suction filtration. The target compound was obtained by recrystallization from ethanol. After drying, 0.0686 g of dark green crystals was obtained. Thin plate chromatography showed no by-products, and the crude yield was 67%. Proton spectrum data: 1 H NMR (400MHz, DMSO-d6) δ8.73 (d, J=8.3Hz, 1H), 8.09–7.97 (m, 3H), 7.86–7.79 (m, 3H), 7.77 (s, 1H), 7.75 ( d,J=7.3Hz,1H),6.82(d,J=8.5Hz,2H),4.07(s,3H),3.91–3.86(m,4H),3.69(t,J=4.5Hz,4H), 3.05(s,6H). See attached for mass spectrometry data figure 1 . The reaction proces...
Embodiment 3
[0049] Embodiment 3: the synthesis of compound B2
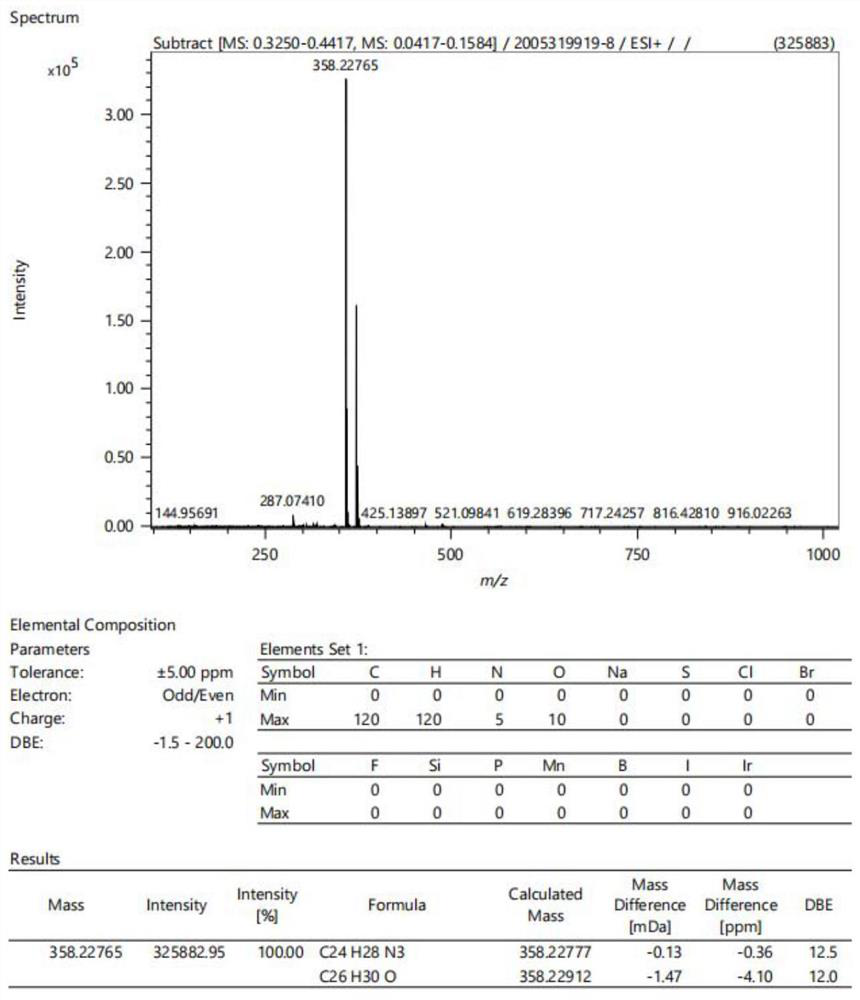
[0050] Intermediate a (0.2 mmol), pyrrolidine (0.6 mmol) and 4-dimethylaminobenzaldehyde (0.4 mmol) were added to the flask. The compound was dissolved with n-butanol (4 mL). The mixture was stirred and refluxed at 80 °C for 8 h. When the reaction was complete, the mixture was cooled to room temperature, then ethyl acetate (10 mL) was added. After standing still for 30 min, the crude product was obtained by suction filtration. The target compound was obtained by recrystallization from ethanol. Drying gave 0.0701 g of orange crystals, thin plate chromatography showed no by-products, and the crude yield was 70%. The hydrogen spectra data are 1 H NMR (400MHz, DMSO-d6) δ8.55 (d, J = 7.9Hz, 1H), 7.95–7.93 (m, 1H), 7.93–7.91 (m, 1H), 7.83 (d, J = 15.8Hz, 1H),7.77–7.74(m,2H),7.71(d,J=15.9Hz,1H),7.63(ddd,J=8.2,5.2,2.9Hz,1H),7.59(s,1H),6.82–6.78 (m, 2H), 3.99 (s, 3H), 3.94–3.88 (m, 4H), 3.03 (s, 6H), 2.03 (p, J=3.4Hz, 4H). See...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com