Application of ingenol compound and derivative thereof in anti-HIV treatment
A compound and composition technology, applied in the field of medicine, can solve the problems of high toxicity and side effects, unsatisfactory, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0254] Embodiment 1 Preparation of ingenol compound EK-5A of the present invention
[0255] Gansui (E. kansui) root and rhizome 5Kg were extracted twice with 8 times the amount of 95% ethanol under reflux, each time for two hours, and the obtained extract was recovered until it had no alcohol smell to obtain an extract. Add water to dilute to 1000mL, extract three times with an equal volume of dichloromethane, recover the dichloromethane solvent, and obtain 101.2g of dichloromethane extract.
[0256] The conditions of the silica gel column chromatography: the mobile phase used is a petroleum ether-ethyl acetate solution with a volume ratio of 10-2:1. The conditions of the reversed-phase chromatography purification: the filler used is octadecyl bonded silica gel, the mobile phase used is isocratic elution with a volume ratio of 90% methanol-water, and the compound EK-5A ( 80mg), light yellow viscous solid, UV absorption 268nm.
[0257] The separation and purification include:...
Embodiment 2
[0260] Example 2 Preparation of Ingenol compound EK-1A of the present invention
[0261] The roots and rhizomes of E. kansui 5Kg were extracted twice with 8 times the amount of 95% ethanol, respectively, for two hours each time. Add water to dilute to 1000 mL, extract three times with equal volume of dichloromethane, and recover the dichloromethane solvent to obtain 101.2 g of dichloromethane extract.
[0262] The conditions of the silica gel column chromatography: the mobile phase used is a petroleum ether-ethyl acetate solution with a volume ratio of 10-2:1. The condition of described reversed-phase chromatography purification: the filler adopted is octadecyl bonded silica gel, and the mobile phase adopted is that the volume ratio is the isocratic elution of the solution of 86% methanol-water, and the compound EK-1A (15 minutes) is obtained. 30mg), pale yellow viscous solid, UV absorption 268nm.
[0263] The separation and purification includes: after concentrating the ext...
Embodiment 3
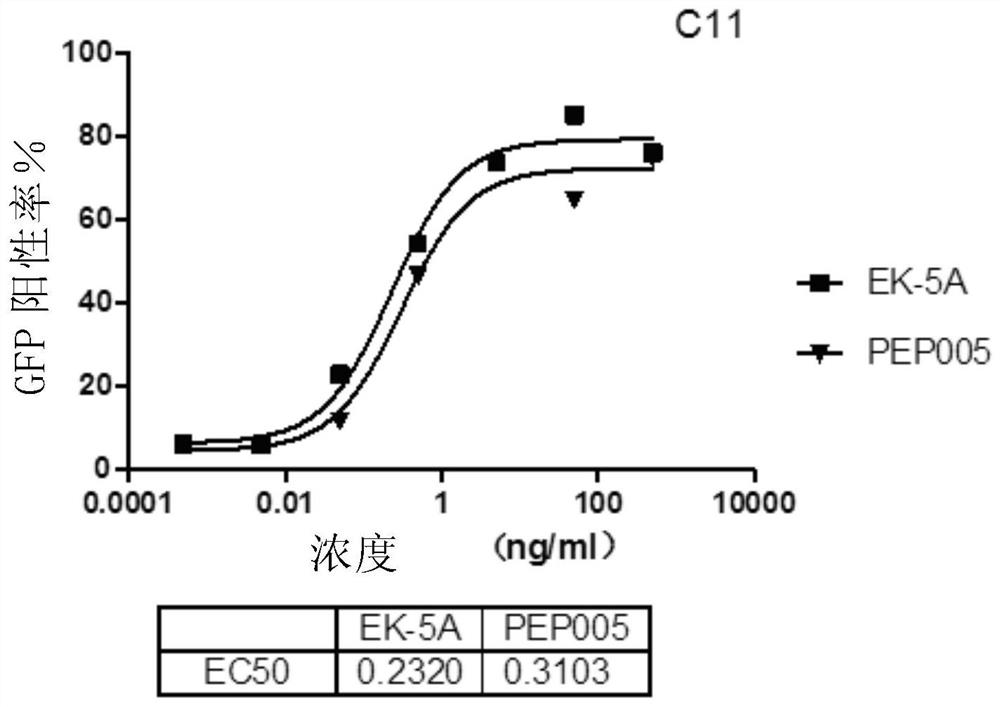
[0265] Example 3 Ingenol compounds of the present invention and derivatives thereof efficiently activate the expression of HIV-1 in latent cells
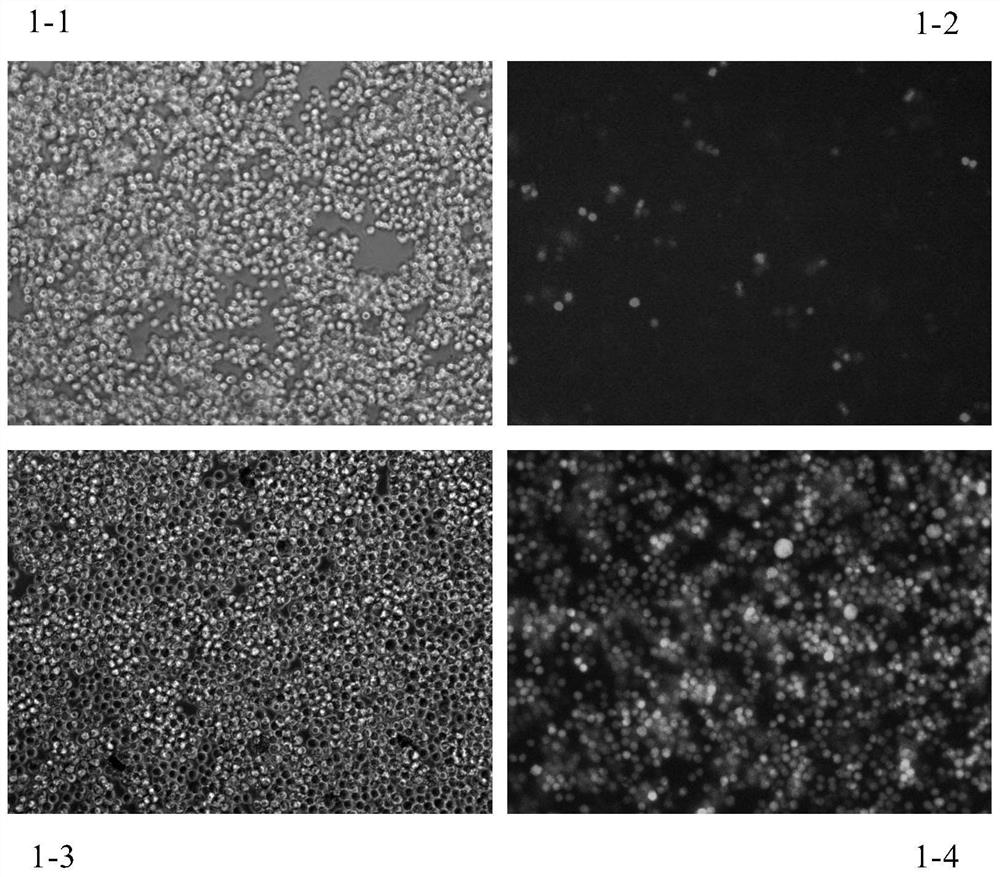
[0266] J-Lat-A10.6 or C11 cells were seeded in a 96-well plate at 2×10E4 cells per well, and 100 μl of 1640 medium (Gibco) containing 10% FBS (Gibco) was added to each well. After the cells were treated with the compounds of Examples 1 and 2 for 48 hours, the green fluorescence expression of the cells was observed under a fluorescence microscope, and the cells were collected for flow cytometry detection to analyze the proportion of fluorescent cells.
[0267] The result is as figure 1 and figure 2 shown. The results showed that the proportion of HIV-positive cells in the latently infected cells treated with no inducer was only about 1-2% of the background activation ( Picture 1-1 and Figure 1-2 ). After treatment with 5ng / ml EK-5A (ie 5A), the proportion of cells expressing green fluorescence in the cell model increased sign...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com