Small molecule drug-loaded polymer vesicles and their preparation method and application
A drug-loaded polymer and polymer technology, applied in drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of low hydrophilic drug loading efficiency, limited overall improvement, and low available dose, and achieve good drug entrapment Effect, good polymer biocompatibility, fast release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 Synthesis of polymer N 3 -PEG-P(TMC-DTC)
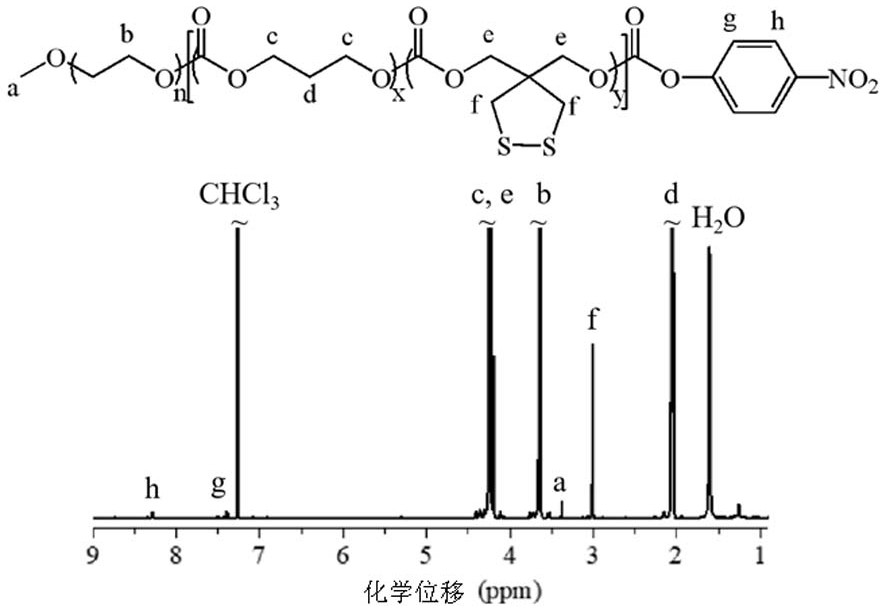
[0054] Polymer N 3 -PEG-P(TMC-DTC) is based on DPP as catalyst, N 3 -PEG-OH is a macromolecular initiator, and it is obtained by initiating ring-opening copolymerization of TMC and DTC. First, weigh N under nitrogen in the glove box 3 -PEG-OH ( M n = 7.9 kg / mol, 0.79 g, 0.1 mmol), TMC (1.50 g, 14.8 mmol) and DTC (0.20 g, 1.0 mmol) in a closed reactor, add 5.0 mL of anhydrous DCM to dissolve, then add DPP (0.25 g , 1.2 mmol), and sealed the reactor, transferred it out of the glove box, and placed it at 30ºC for four days. After the reaction, it was precipitated twice with glacial ether and dried in vacuo to obtain a white flocculent polymer N. 3 -PEG-P(TMC-DTC), yield: 85.4%. attached figure 1 N at δ 3.38 and 3.63 ppm can be seen in 3 - Characteristic peaks for PEG, characteristic peaks for TMC at δ 2.03 and 4.18 ppm, and characteristic peaks for DTC at δ 2.99 and 4.22 ppm. N can be calculated from the ratio...
Embodiment 2
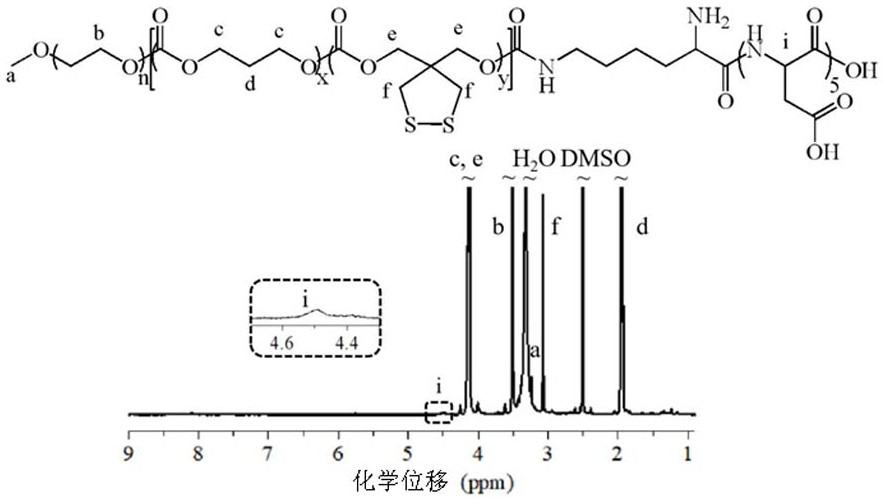
[0056] Example 2 Synthesis of polymer PEG-P(TMC-DTC)-KD z
[0057] Polymer PEG-P(TMC-DTC)-KD z The synthesis is divided into two steps, that is, using p-NPC to activate the terminal hydroxyl group of PEG-P(TMC-DTC) (5.0-(15.0-2.0) kg / mol), and then react with KD z Polypeptide molecules are reacted. PEG-P(TMC-DTC)-KD 5The synthesis of PEG-P(TMC-DTC) (1.0 g, 45.5 μmol) was dissolved in 10 mL of anhydrous DCM under nitrogen atmosphere, and then transferred to an ice-water bath and added with pyridine (18.0 mg, 45.5 μmol). 227.5 μmol), and after stirring for 10 minutes, a solution of p-NPC (48.4 mg, 240.3 μmol) in DCM (1.0 mL) was added dropwise. After the completion of the dropwise addition in 30 minutes, the reaction was continued for 24 hours at room temperature, and then the pyridinium salt was removed by suction filtration, and the polymer solution was collected by rotary evaporation and concentrated to ~100 mg / mL, precipitated with glacial ether, and dried in vacuo to ob...
Embodiment 3
[0058] Example 3 Preparation of VCR-loaded reversibly cross-linked biodegradable vesicles (Ps-VCR)
[0059] Ps-VCR was prepared by a solvent displacement method, in which VCR was synthesized with KD z The electrostatic interaction between them is encapsulated. PEG-P(TMC-DTC)-KD z Dissolve in DMSO (40 mg / mL), dispense 100 µL into standing 900 µL HEPES (pH 6.8, 10 mM) containing VCR, stir at 300 rpm for 3 minutes, and add HEPES (pH 7.4, 10 mM) ) Ps-VCR was obtained by dialysis for 8 hours. The theoretical drug loading of VCR was set at 4.8-11.1 wt.%, and it was found that the particle size of the obtained Ps-VCR was between 26-40 nm and the particle size distribution was 0.05-0.20 (Table 1). The encapsulation efficiency of Ps-VCR was calculated as high as 97.2% by measuring its absorbance at 298 nm by UV-Vis spectroscopy. Based on the same method, under the theoretical drug loading of 4.8%, PEG-P(LA-DTC)-KD 5 , PEG-P(CL-DTC)-KD 5 The encapsulation efficiencies of the prepa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com