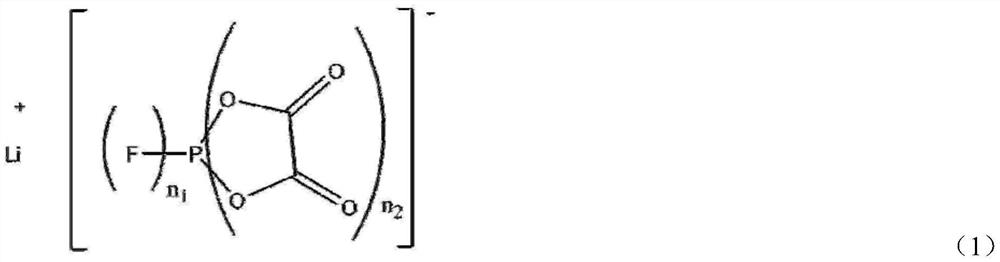
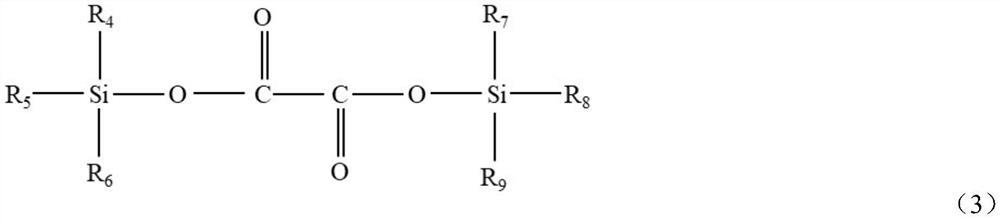
Synthetic method of oxalate lithium phosphate salt compound
A salt compound, lithium phosphate technology, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve problems such as adverse effects on the performance of non-aqueous electrolyte batteries and difficulty in industrialization, Achieve the effects of low cost, high reaction yield and simple reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Add 0.124mol ammonium hexafluorophosphate, 0.254mol bistrimethylsilyl oxalate, 0.150mol lithium hydride and anhydrous diethyl carbonate to a 500ml round bottom flask in a glove box, and react at 80°C for 24h until Gas is no longer produced. The solvent was then removed to obtain a white solid with a yield of 94%. NMR test results show that the ratio of LiDFBOP:LiTFOP is 72:1, and the molar percentage of LiDFBOP is 98.6%.
Embodiment 2
[0040] Add 0.124mol ammonium hexafluorophosphate, anhydrous diethyl carbonate, and 0.273mol bistrimethylsilyl oxalate to a 500ml round bottom flask in a glove box, and react at 100°C for 1h until no gas is produced . Then add 0.118mol lithium hydride to continue the reaction, and react at 100°C for 1h. The solvent was then removed to give a white solid with a yield of 89%. NMR test results show that the ratio of LiDFBOP:LiTFOP is 20:1, and the molar percentage of LiDFBOP is 95.2%.
Embodiment 3
[0042] Same as Example 2, except that the addition amounts of ammonium hexafluorophosphate and bistrimethylsilyl oxalate are 0.124mol and 0.254mol respectively, and the product yield is 88%. The NMR test results show that the ratio of LiDFBOP:LiTFOP is 72:1, the molar percentage of LiDFBOP is 98.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com