Synthesis and photoelectric property research of carbazole room-temperature phosphorescent material containing S/Se/Te heavy atoms
一种咔唑基、化合物的技术,应用在咔唑类室温磷光材料的合成领域,能够解决自猝灭、氧猝灭等问题,达到合成条件温和、窄光学带隙、满足测试要求的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
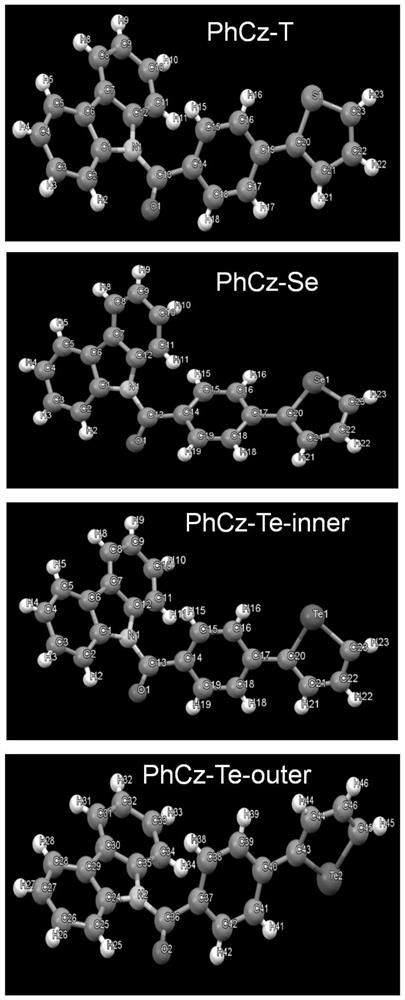
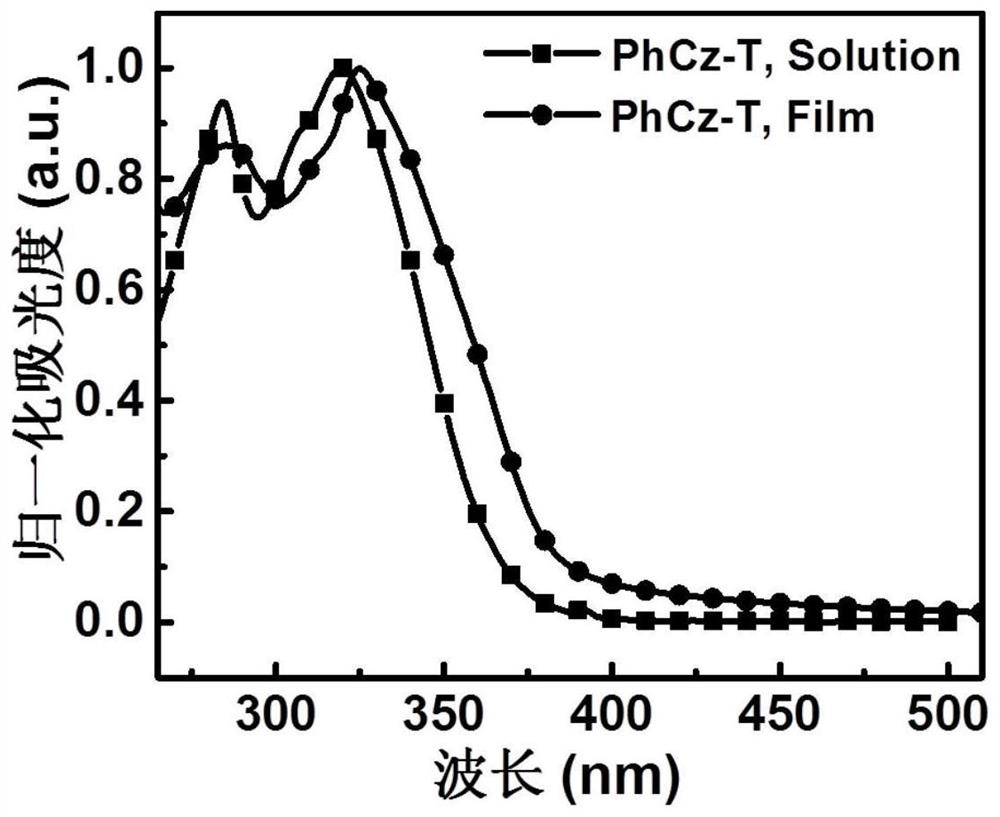
[0055] A D-A type carbazolyl compound PhCz-T of the present invention has a general structural formula of formula I:
[0056]
[0057] The synthetic route of PhCz-T is:
[0058]
[0059] Specifically include the following steps:
[0060] (1) Synthesis of intermediate a: Add carbazole (1.0 g, 6.0 mmol) and anhydrous tetrahydrofuran (30 mL) to 100 mL of Schlenk under nitrogen protection, and then place the reaction bottle in a cryogenic apparatus at -78°C. At -78°C, lithium diisopropylamide (2.0M, 3.3mL) was slowly added dropwise into the reaction flask, reacted at -78°C for 1h, and then slowly returned to -40°C and stirred for 1h. Then, a tetrahydrofuran solution (2.4 g, 9 mmol) of 4-iodobenzoyl chloride was injected once at −78° C., and finally stirred overnight at room temperature. The mixture was extracted with ethyl acetate and water, dried, filtered and spin-dried. The crude product was purified by silica gel column chromatography (petroleum ether: ethyl acetate =...
Embodiment 2
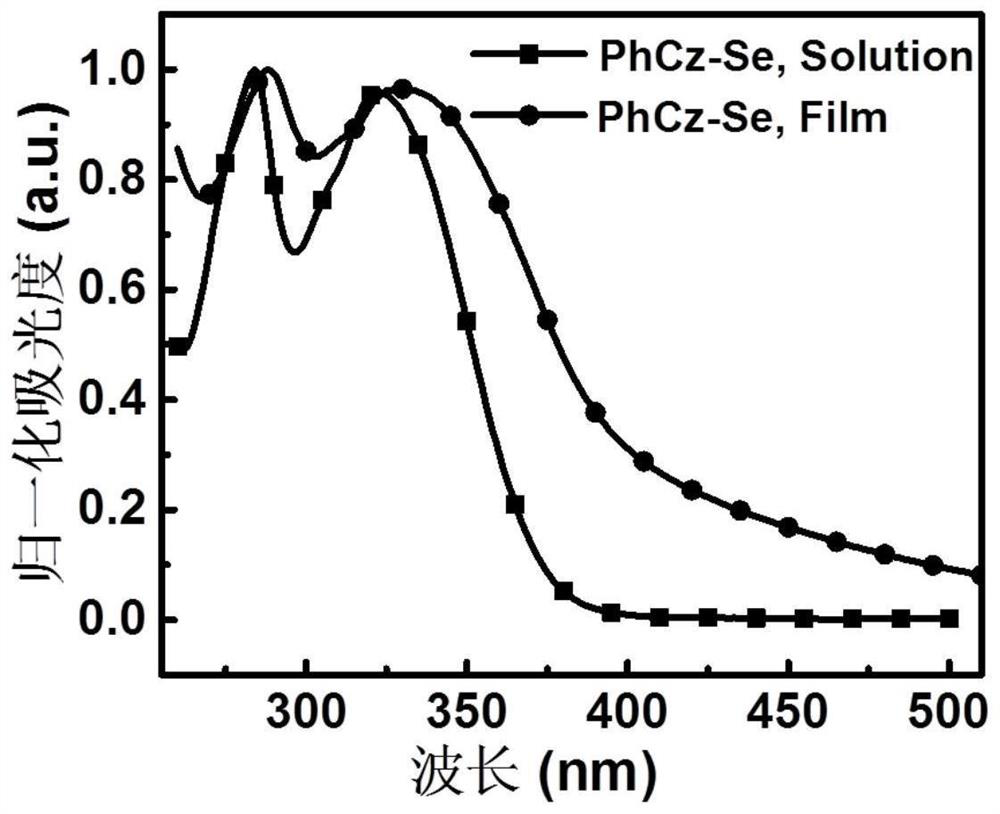
[0077] A D-A type carbazolyl compound PhCz-Se of the present invention has a general structural formula of formula II:
[0078]
[0079]
[0080] The synthetic route of PhCz-Se is:
[0081]
[0082] Concrete synthetic steps are:
[0083] (1) Synthesis of intermediate a: synthesized with reference to the synthetic method of the above-mentioned Example 1.
[0084] (2) Synthesis of compound PhCz-Se: In a 10 mL microwave bottle, add iodocarbazolyl intermediate (0.85 g, 2.1 mmol), selenophene unilateral tin compound (1.0 g, 3.4 mmol) and 6 mL of toluene. Oxygen was replaced under nitrogen for 20 minutes, then the catalyst tetrakistriphenylphosphine palladium (8 mg) was added, nitrogen was blown for 10 minutes, a microwave cover was sealed, and the reaction was carried out at 120° C. for 4 hours. After cooling to room temperature, extract with dichloromethane, dry over anhydrous magnesium sulfate, filter with suction, and spin dry. The mixture was purified by silica gel ...
Embodiment 3
[0094] A D-A type carbazolyl compound PhCz-Te of the present invention has a general structural formula of formula III:
[0095]
[0096] The synthetic route of PhCz-Te is:
[0097]
[0098] Concrete synthetic steps are:
[0099] (1) Synthesis of intermediate a: synthesized with reference to the synthetic method of the above-mentioned Example 1.
[0100] (2) Synthesis of compound PhCz-Te: In a 10mL microwave bottle, put iodocarbazolyl intermediate (0.8g, 2.0mmol), tellurphene unilateral tin compound (1.0g, 3.0mmol) and 6mL chlorobenzene . Oxygen was replaced under nitrogen for 20 minutes, then the catalyst tetrakistriphenylphosphine palladium (8 mg) was added, nitrogen was blown for 10 minutes, a microwave cover was sealed, and the reaction was carried out at 140° C. for 4 hours. After cooling to room temperature, extract with dichloromethane, dry over anhydrous magnesium sulfate, filter with suction, and spin dry. The mixture was purified by silica gel column chroma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal degradation temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com