(z)-β-trifluoromethyl dehydrotryptophan compound and its synthesis method and application
The technology of a trifluoromethyl group and a synthesis method, which is applied in the field of organic synthesis, can solve the problems of poor atom economy, high operation requirements, long synthesis steps and the like, and achieves the effects of improving biological activity, high application value and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
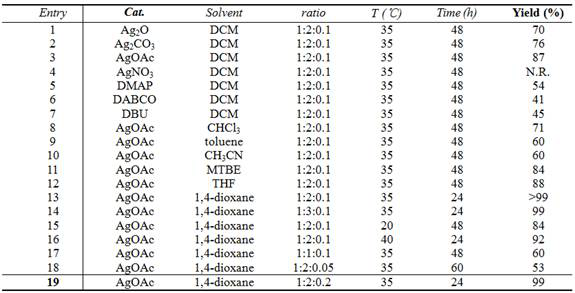
[0031] Catalyst screening experiments: Different catalysts promote the reaction of 3-trifluoroacetyl indole with isocyanate, resulting in different yields.
[0032] Pick N - p-toluenesulfonyl-3-trifluoroacetylindole (0.2 mmol), methyl isocyanate (0.4 mmol), catalyst (0.02 mmol), added to 2 mL of dichloromethane, 35 ° C for 48 hours, the reaction After completion, the solvent was removed under reduced pressure, and compound A was obtained by column chromatography.
[0033]
[0034]
[0035] The ratio in the table refers to the N - p-toluenesulfonyl-3-trifluoroacetylindole:methyl isocyanate:catalyst ratio.
Embodiment 2
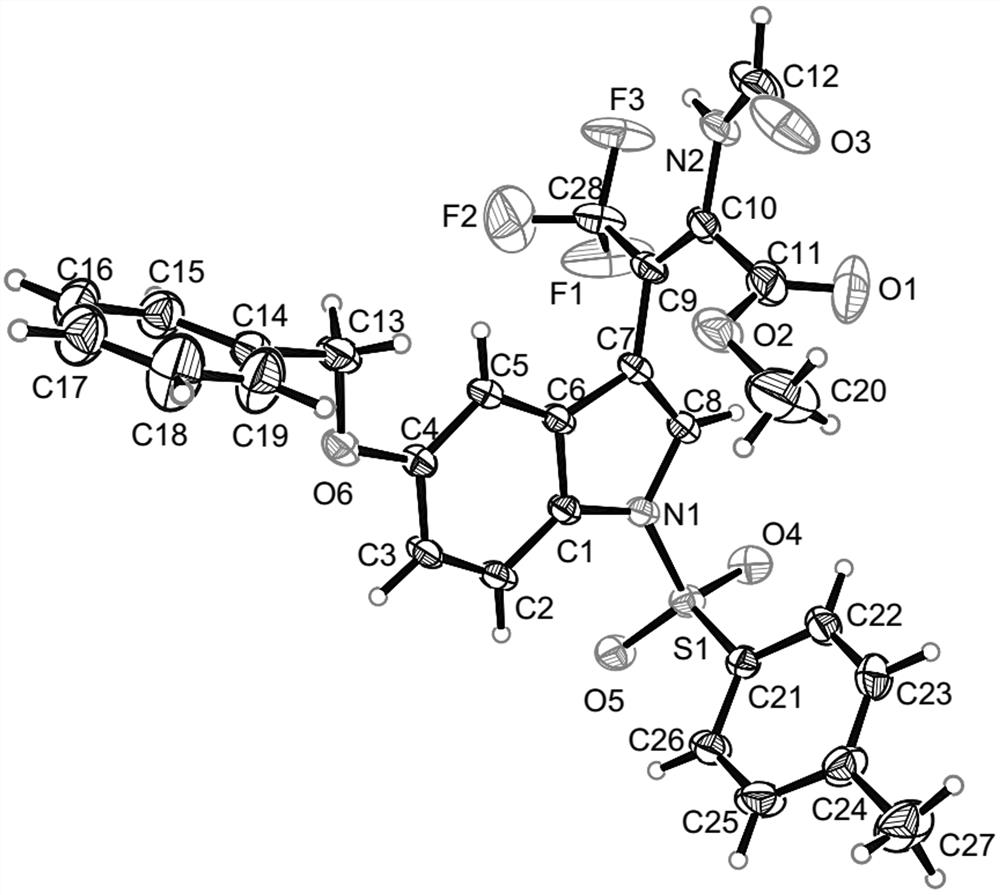
[0037] The preparation method of compound A with the following structure:
[0038]
[0039] Pick N - p-toluenesulfonyl-3-trifluoroacetylindole (0.2 mmol), methyl isocyanate (0.4 mmol), AgOAc (0.02 mmol), added to 2 mL 1,4-dioxane, 35 °C The reaction was carried out for 24 hours. After the reaction was completed, the solvent was removed under reduced pressure, and 92.3 mg of compound A was obtained as a white solid through column chromatography separation, with a yield of >99%.
[0040] NMR characterization is as follows: 1 H NMR (400 MHz, CDCl 3) δ 8.27 (s, 1H), 8.14 (s, 1H),7.97 (d, J = 8.3 Hz, 1H), 7.77 (d, J = 8.2 Hz, 2H), 7.58 (s, 1H), 7.44 (d, J = 7.8 Hz, 1H), 7.34 (d, J = 7.8 Hz, 1H), 7.26 (t, J = 7.1 Hz, 3H), 3.19 (s,3H), 2.34 (s, 3H); 13 C NMR (100 MHz, CDCl 3 ) δ 162.9, 158.0, 145.4, 134.9, 134.4, 134.3, 130.0, 130.0, 127.5, 126.9, 125.4, 123.9, 123.2 (q, J = 275.1Hz, 1C), 120.4, 113.5, 112.2, 108.9 (q, J = 32.6 Hz, 1C), 52.4, 21.6.
Embodiment 3
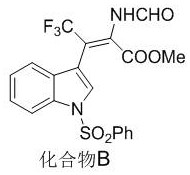
[0042] The preparation method of compound B with the following structure:
[0043]
[0044] Pick N -Phenylsulfonyl-3-trifluoroacetylindole (0.2 mmol), methyl isocyanate (0.4 mmol), AgOAc (0.02 mmol), add 2 mL of 1,4-dioxane, and react at 35°C After 24 hours, after the reaction was completed, the solvent was removed under reduced pressure, and 87.7 mg of compound B was obtained by column chromatography separation as a colorless oil, with a yield of 97%.
[0045] NMR characterization is as follows: 1 H NMR (400 MHz, CDCl 3 ) δ 8.20 (s, 1H), 7.96 (s, 1H), 7.92 (d, J = 8.3 Hz, 1H), 7.85-7.78 (m, 2H), 7.50 (s, 1H), 7.48 (d, J = 7.4Hz, 1H), 7.39 (dd, J = 16.9, J = 8.4 Hz, 3H), 7.28 (d, J = 7.8 Hz, 1H), 7.24-7.19 (m, 1H), 3.07 (s, 3H); 13 C NMR (100 MHz, CDCl 3 ) δ162.8, 157.8, 137.9,134.5, 134.3, 134.2, 130.0, 129.5, 127.5, 126.9, 125.5, 124.0, 123.2 (q, J =275.1 Hz, 1C), 120.5, 113.5, 112.4, 108.7 (q, J = 33.5 Hz, 1C), 52.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com