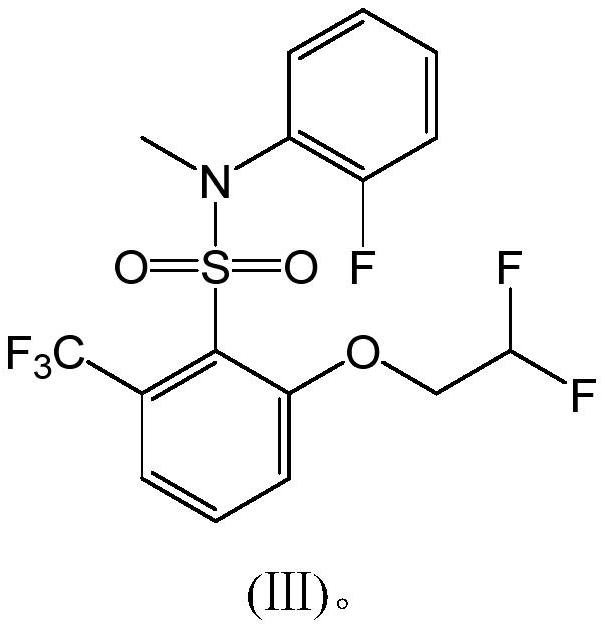
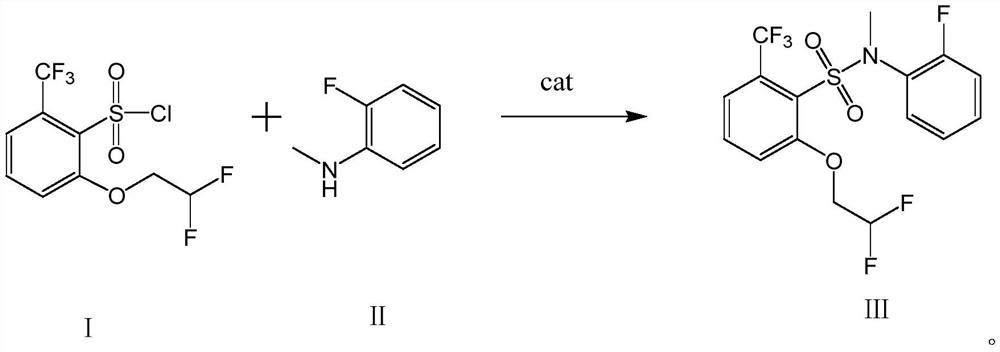
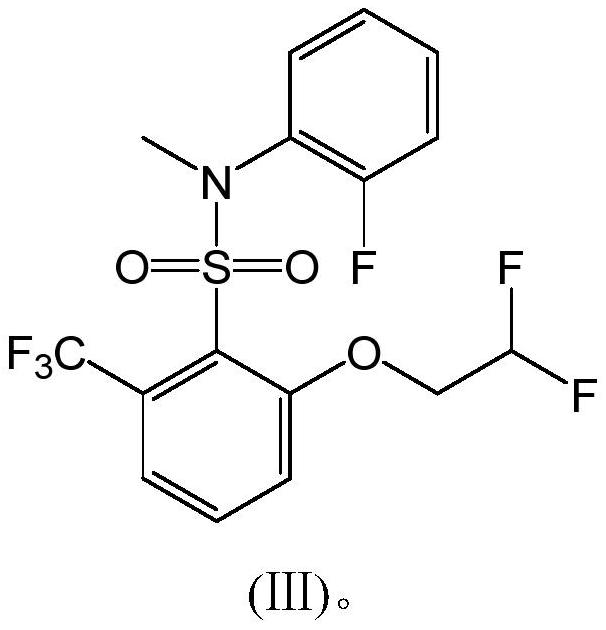
A compound containing hexafluorosulfonamide structure with herbicidal properties and its synthesis method
A synthesis method and compound technology, which are applied in the fields of sulfonic acid amide preparation, botanical equipment and methods, organic chemistry, etc., can solve the problem of no obvious herbicidal effect of Fructus chinensis, and achieve good herbicidal effect, small dosage and wide herbicidal spectrum. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 460g of toluene and 162.3g of compound I into a 1000ml three-necked flask, and stir for 30min at a temperature of 60-65°C to dissolve compound I in toluene.
[0034] Add 62.5g of compound II, 160.7g of 3,5-lutidine, and 2.5g of DMSO into a 2000ml three-necked flask, stir, raise the temperature to 65°C, and add the toluene solution of compound I dropwise to the mixture containing compound II within about 2 hours. solution, keep warm for 2h after the dropwise addition. Reaction finishes, and reaction solution is cooled to 10 ℃, and hydrochloric acid (concentration is 10%) is added dropwise to adjust pH value to 2, layering, organic phase rotary steaming, adds 500g concentrated hydrochloric acid (concentration is 30%), 0~10 ℃ of crystallization, Filter, wash with water until the pH value reaches 7, filter, and dry to obtain 195 g of the finished product (Compound III, white solid), with a content of 98.3% and a yield of 94.36%.
[0035] 1 H NMR (DMSO-d 6 ,300MHz) δ:...
Embodiment 2
[0037] Add 500g of toluene and 162.3g of compound I into a 1000ml three-neck flask, keep the temperature at 60-65°C and stir for 30min to dissolve compound I in toluene.
[0038] Add 62.5g of compound II, 160.7g of 3,5-lutidine, and 2.5g of DMSO into a 2000ml three-necked flask, stir, raise the temperature to 65°C, and add the toluene solution of compound I dropwise to the mixture containing compound II within about 2 hours. solution, keep warm for 2h after the dropwise addition. Reaction finishes, and reaction solution is cooled to 10 ℃, and hydrochloric acid (concentration is 10%) is added dropwise to adjust pH value to 2, layering, organic phase rotary steaming, adds 500g concentrated hydrochloric acid (concentration is 30%), 0~10 ℃ of crystallization, Filter, wash with water until the pH value reaches 7, filter, and dry to obtain 194 g of the finished product, with a content of 98.2% and a yield of 93.87%.
Embodiment 3
[0040] Add 460g of toluene and 162.3g of compound I into a 1000ml three-neck flask, keep the temperature at 60-65°C and stir for 30min to dissolve compound I in toluene.
[0041]Add 62.5g of compound II, 107g of 3,5-lutidine, and 2.5g of DMSO into a 2000ml three-necked flask, stir, raise the temperature to 65°C, and add the toluene solution of compound I to the mixture containing compound II dropwise within about 2 hours After the dropwise addition, keep warm for 2h. Reaction finishes, and reaction solution is cooled to 10 ℃, and hydrochloric acid (concentration is 10%) is added dropwise to adjust pH value to 2, layering, organic phase rotary steaming, adds 500g concentrated hydrochloric acid (concentration is 30%), 0~10 ℃ of crystallization, Filter, wash with water until the pH value reaches 7, filter, and dry to obtain 163 g of the finished product, with a content of 96.5% and a yield of 78.89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com