Vaccine for use in prophylaxis and/or treatment of disease
A technology for vaccines and diseases, applied in the field of vaccines, can solve problems such as
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0359] Mutations in the vaccine-encoded immunosuppressive domain (ISD)
[0360] As an improved first strategy, two point mutations were introduced in the sequence of MelARV Env to inactivate the immunosuppressive domain (ISD) ( image 3 ). These specific mutations were previously tested and analyzed for murine leukemia virus by Schlecht-Louf et al. (Schlecht-Louf, G. et al., Retroviral infection in vivo requires an immune escape virus factor encrypted in the envelope protein of oncoretroviruses. Proc Natl Acad Sci U S A, 2010. 107(8): pp. 3782-7). The virus encoding this modified form of MelARV Env is called Ad5-MelARV-ISD.
[0361] Effect of Ad5-MelARV-ISD on antibody responses in CD1 mice
[0362] Outbred CD1 mice were primed with DNA-MelARV or DNA-MelARV-ISD and subsequently boosted with AD5-MelARV or Ad5-MelARV-ISD according to vaccination timeline IV. Four weeks after adenovirus vaccination, blood samples were collected and analyzed by ELISA.
[0363] like Fi...
Embodiment 2
[0367] Effect of Ad5-MelARV-ISD on antibody response and metastasis in C57BL / 6 mice
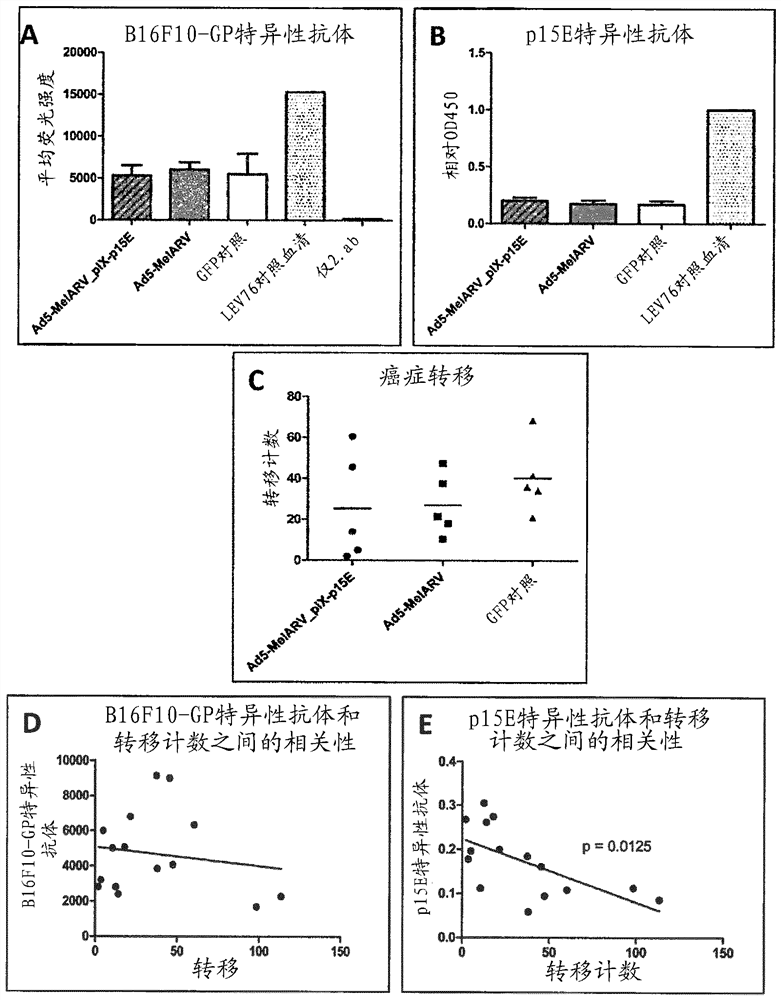
[0368]C57BL / 6 mice were vaccinated and challenged according to vaccination timeline III. Mice received DNA-MelARV or DNA-MelARV-ISD followed by the corresponding adenovirus. Analysis of antibody responses revealed that MelARV-ISD slightly increased the level of B16F10-GP cell-specific antibodies ( Figure 8A ). However, this increase was not significant and was only slightly above background in PBS-vaccinated mice. Such as Figure 8B As shown in , no effect was observed for antibodies specific for p15E. Corresponding to tumor cell-bound antibodies, metastasis was slightly, but not significantly, reduced in MelARV-ISD vaccinated mice ( Figure 8C )
Embodiment 3
[0370] Effect of Ad5-MelARV-ISD on T cell responses in Balb / C mice
[0371] In addition to antibody responses, the effect of Ad5-MelARV-ISD on T cell priming and activation was also analyzed. ELISPOT ( Figure 9 ) and ICS (Fig. 10) both showed increased levels of AH1-specific T cells in Ad5-MelARV-ISD vaccinated mice compared to Ad5-MelARV. As observed by ICS, double positive IFNγ compared with the native form + TNFα + CD8 + T cells were significantly increased in Ad5-MelARV-ISD vaccinated mice. IFNγ + The integrated geometric mean (IGM) of the cells also showed significant differences from native Ad5-MelARV. The IGM combines the number of positive cells with the mean fluorescence intensity and thus also takes into account the quality of activated immune cells. The IGM of TNFa remained insignificant (data not shown).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com