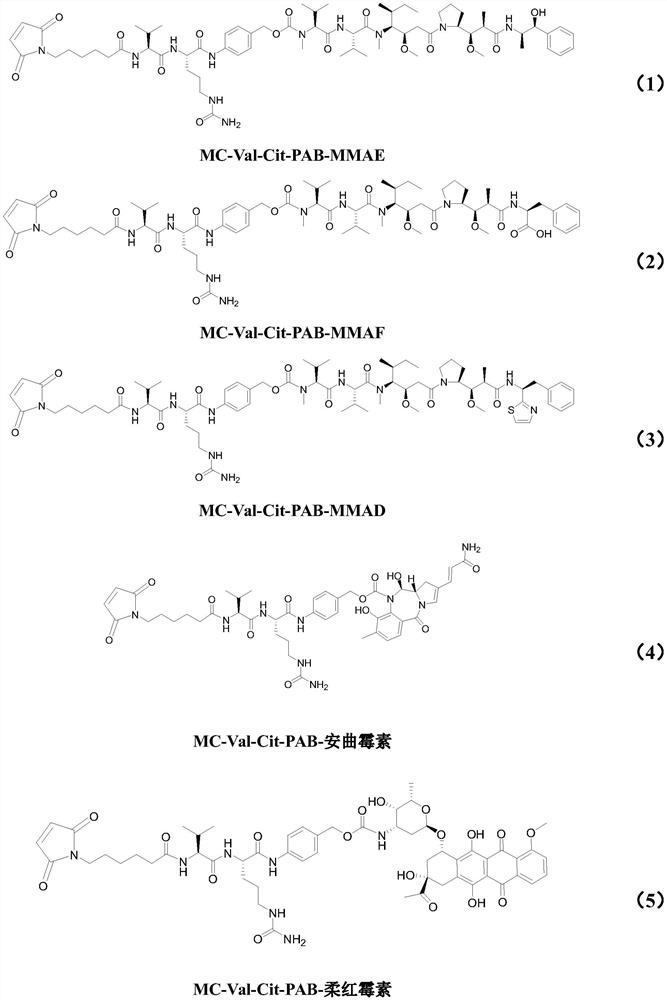
Preparation technology of antibody drug conjugate intermediate by one-pot method
A preparation process and technology for antibody drugs, which are applied in the preparation methods of peptides, anti-tumor drugs, drug combinations, etc., can solve the problems of high material loss, high price, and increased production costs in the production of drugs, so as to reduce production costs and reduce The effect of producing waste liquid and improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The preparation of embodiment 1Py-MAA-Val-Cit-PAB-MMAE
[0051] (1) "One pot method" preparation
[0052]
[0053] Add Py-MAA-Val-Cit-PAB-OH (1.8g, 1.0eq) and DMF (40mL) successively to a three-neck round bottom flask, stir until dissolved, then add NPC (882mg, 1.1eq), DIPEA (336mg, 1.0eq), stirred at 24±2°C for 24 hours. Then, DIPEA (672mg, 2.0eq), pyridine (2.3mL), HoBt (351mg, 1.0eq) and MMAE (1.7g, 0.9eq) were added successively to the reaction solution, and the reaction was continued at 24±2°C for 48 hours to prepare the solution The product Py-MAA-Val-Cit-PAB-MMAE (1.9g) was obtained by phase purification, with a purity of 99.84% and a yield of 51.3% [calculation formula: yield=Py-MAA-Val-Cit-PAB-MMAE output ÷(Py -MAA-Val-Cit–PAB-OH dosage ÷702.8×1446.8)×100%].
[0054] (2) "Two-step method" preparation
[0055] Step 1: Preparation of Py-MAA-Val-Cit-PAB-(4-nitrophenyl)carbonate
[0056]
[0057] In the reaction flask, add DMF (40mL), Py-MAA-Val-Ci...
Embodiment 2
[0062] Example 2 Preparation of Py-MAA-Val-Cit-PAB-MMAD
[0063] (1) "One pot method" preparation
[0064]
[0065] Into the reaction bottle, add DMF (4mL), Py-MAA-Val-Cit-PAB-OH (200mg, 1.0eq.), stir to dissolve and add bis(p-nitrophenyl)carbonate (NPC, 95mg, 1.1eq .) and DIPEA (36mg, 1.0eq.), react at 24±2°C for 24 hours, add 1-hydroxybenzotriazole (HoBt, 38mg, 1.0eq.), MMAD (197mg, 0.9eq.) , pyridine (248μL) and DIPEA (73mg, 2.0eq), reacted at 24±2°C for 48 hours, spin-dried, and prepared Py-MAA-Val-Cit-PAB-MMAD (208mg) by HPLC, yield: 48.7%, Purity: 99%.
[0066] (2) "Two-step method" preparation
[0067] Step 1: Preparation of Py-MAA-Val-Cit-PAB-(4-nitrophenyl)carbonate
[0068]
[0069] Into the reaction bottle, add DMF (4mL), Py-MAA-Val-Cit-PAB-OH (200mg, 1.0eq.), stir to dissolve and add bis(p-nitrophenyl)carbonate (NPC, 95mg, 1.1eq .) and DIPEA (36mg, 1.0eq.), reacted at 24±2°C for 24 hours. Ethyl acetate (6 mL) was added to the reaction liquid, and ...
Embodiment 3
[0074] Example 3 Preparation of Py-MAA-Val-Cit-PAB-DX8951
[0075] (1) "One pot method" preparation
[0076]
[0077]Into the reaction bottle, add DMF (4mL), Py-MAA-Val-Cit-PAB-OH (200mg, 1.0eq.), stir to dissolve and add bis(p-nitrophenyl)carbonate (NPC, 95mg, 1.1eq .) and DIPEA (36 mg, 1.0 eq.). React at 24±2°C for 24 hours, add 1-hydroxybenzotriazole (HoBt, 38mg, 1.0eq.), DX8951 (136mg, 0.9eq.), pyridine (248μL) and DIPEA (110mg, 3.0eq), 24± The reaction was continued at 2°C for 48 hours, spin-dried, and Py-MAA-Val-Cit-PAB-DX8951 (123 mg) was prepared by HPLC with a yield of 37.1% and a purity of 97%.
[0078] (2) "Two-step method" preparation
[0079] Step 1: Preparation of Py-MAA-Val-Cit-PAB-(4-nitrophenyl)carbonate
[0080]
[0081] Into the reaction bottle, add DMF (4mL), Py-MAA-Val-Cit-PAB-OH (200mg, 1.0eq.), stir to dissolve and add bis(p-nitrophenyl)carbonate (NPC, 95mg, 1.1eq .) and DIPEA (36mg, 1.0eq.), reacted at 24±2°C for 24 hours. Ethyl acetat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com