Novel phenolic compound and preparation method and application thereof
A technology of phenolic compounds and compounds, applied in the field of new phenolic compounds and their preparation, can solve the problem of high MIC, and achieve the effect of inhibiting growth and good antibacterial effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] 1. When R=Br or Cl in the novel phenolic compound of formula (1), the preparation method includes:
[0049] Step 1. React 4-hydroxydiphenylmethane with a halogen-containing compound to obtain compound II, and the halogen includes bromine or chlorine; specifically,
[0050] (1) When R=Br, after dissolving 4-hydroxydiphenylmethane in acetic acid solution, bromine gas can be directly introduced for reaction. The halogen-containing compound is bromine gas, and 4-hydroxydiphenylmethane and The molar ratio of bromine gas is preferably in the range of 15-17:32-34, within which the molar ratio can be fully reacted;
[0051] Preferably, an organic solvent is also added to the reaction, the organic solvent oil chloroform, acetic acid, methanol, etc., in this embodiment, AcOH acetic acid is preferred, because according to experiments, it is found that the yield of acetic acid is the highest for this structure.
[0052] (2) When R=Cl, due to the low reaction efficiency of introducing chlor...
Embodiment 1
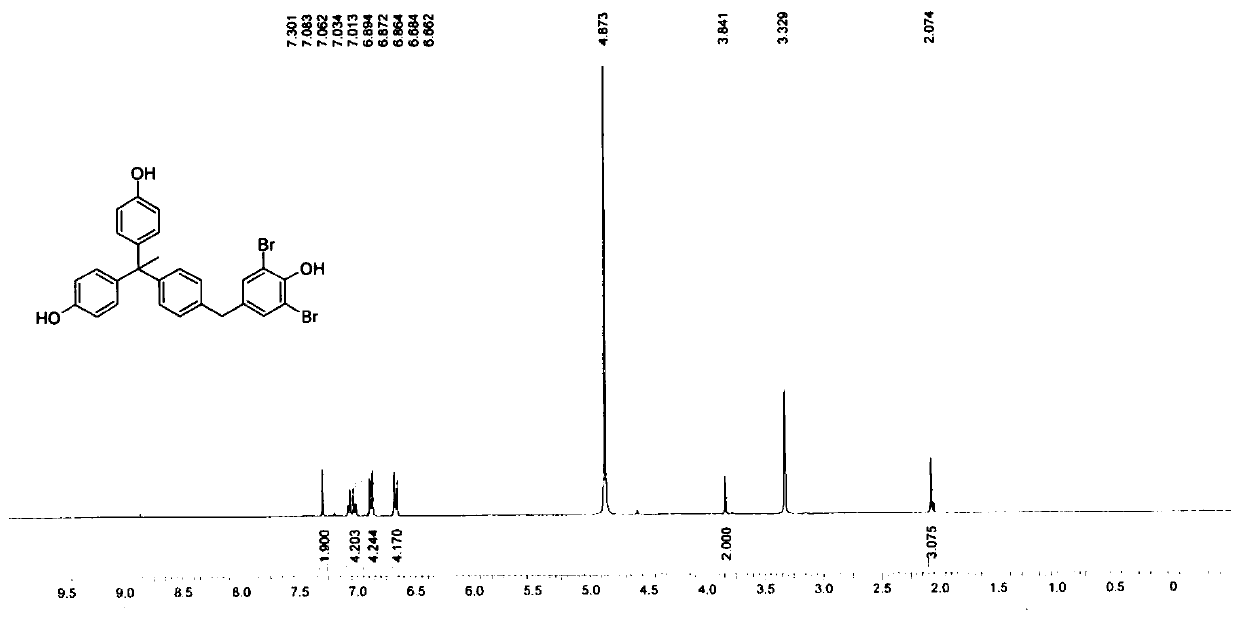
[0082] When R=Br in formula (1), the structural formula of the novel phenolic compound in this embodiment is:
[0083]
[0084] The specific preparation method is as follows:
[0085]
[0086] Step 1. Add compound 1 (4-hydroxydiphenylmethane, 3.00g, 16.3mmol, 1.00eq) to AcOH (15.0mL), add Br dropwise at 10°C 2 (5.23g, 32.7mmol, 1.69mL, 2.01eq). The mixture was stirred at 5-25°C for 2 hours.
[0087] After TLC (petroleum ether: ethyl acetate = 5:1, Rf = 0.59) detects that the 4-hydroxydiphenylmethane reaction is complete, the reaction mixture is poured into twice the volume of ice water and extracted twice with the same volume of ethyl acetate. Use NaHCO for the organic phase 3 Wash twice, then wash with concentrated brine, anhydrous Na 2 SO 4 Dry, filter and concentrate under reduced pressure to obtain the residue, and then purify by column chromatography to obtain a pale yellow oily solution A, to obtain compound II and detect the compound by nuclear magnetic resonance;
[0088] Ste...
Embodiment 2
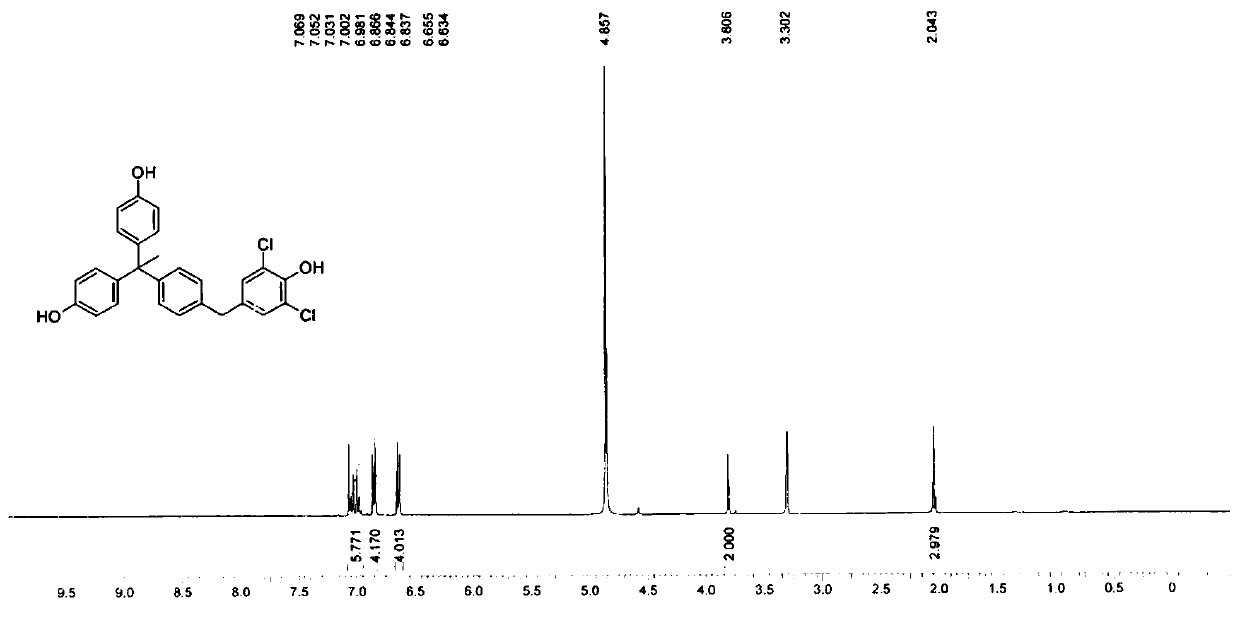
[0092] When R=Cl in formula (1), the structural formula of the novel phenolic compound of this embodiment is:
[0093]
[0094] The specific preparation method is as follows:
[0095]
[0096] Step 1. Add compound 1 (3.00g, 16.3mmol, 1eq) in MeOH (50.0mL) and add NaOH (977mg, 24.4mmol, 1.5eq) and NaCl (2.85g, 48.9mmol, 3eq) to the solution of adding compound 1 (3.00g, 16.3mmol, 1eq), the temperature is 5-15°C to obtain a mixture. NaClO (45.5 g, 48.9 mmol, 37.6 mL, 8% purity, 3 eq) was added dropwise to the mixture, and stirred at 5-15°C for 13 hours.
[0097] TLC (petroleum ether: ethyl acetate = 5:1) indicates that reactant 1 has been completely consumed. The mixture was concentrated under reduced pressure to remove most of the MeOH. Dilute with water (15.0 mL) and extract with EtOAc (20.0 mL*2). The mixed organic layer was washed with brine (15.0 mL), and dried in anhydrous Na 2 SO 4 It was dried, filtered, and concentrated under reduced pressure to obtain a residue. The resid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap