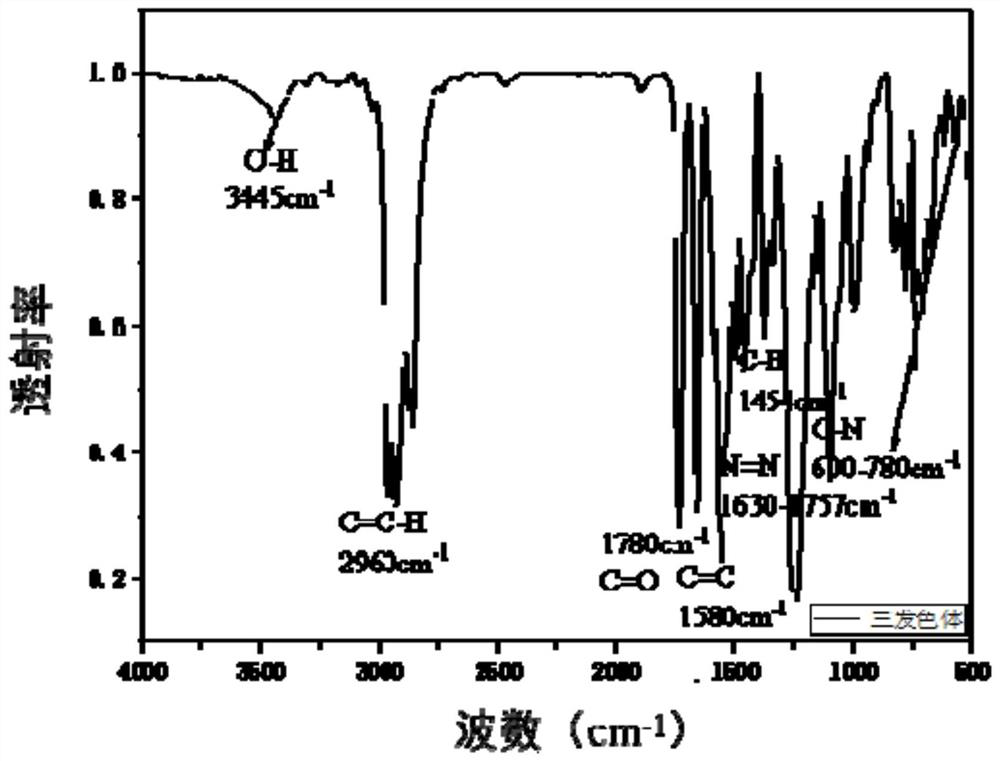
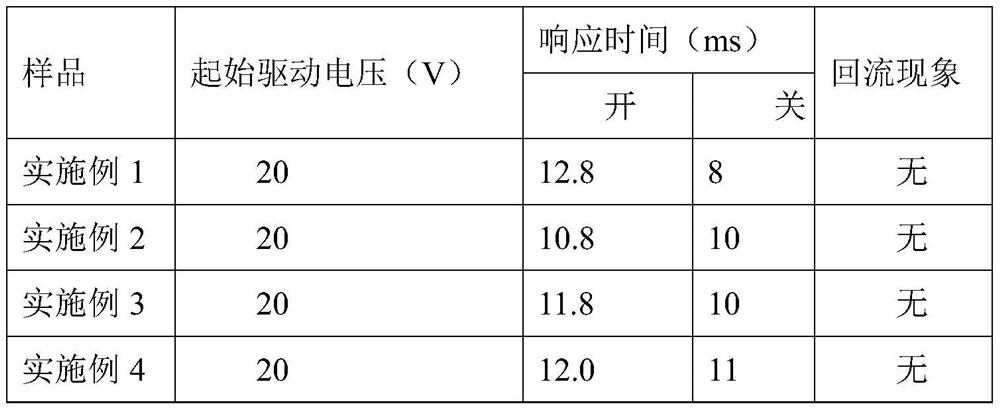
Multichromophoric pyrazolone azo-based dyes, inks and electrowetting displays
A technology of pyrazolone azo and chromophore, which is applied to the preparation of azo dyes, azo dyes, inks, etc., and can solve the problems of unfavorable electrowetting color development, fast fading speed, and poor light stability of dye molecules. problem, achieve the effect of reducing apparent molecular polarity, reducing ink reflow effect, and improving light stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
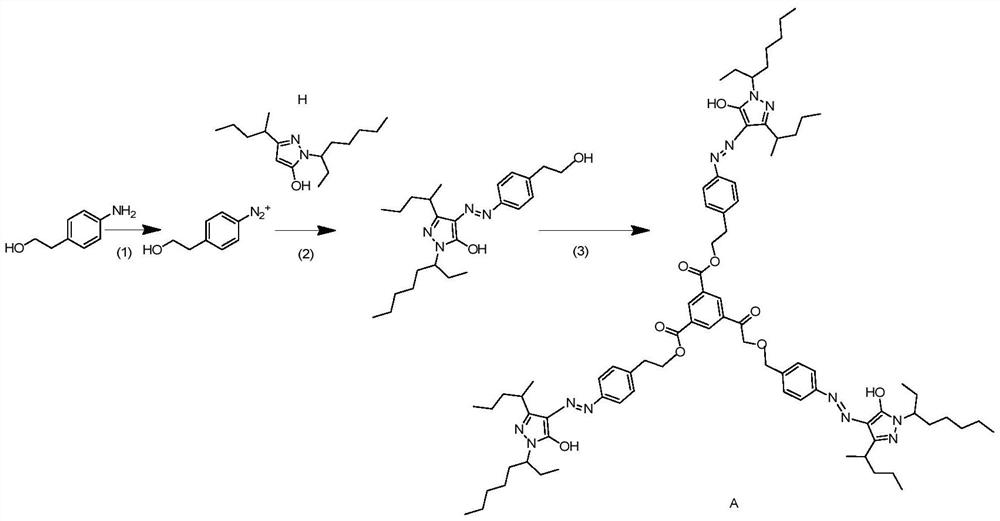
[0026]This embodiment provides a multi-color pyrazolinocardium azo dye A, which synthesis route is
[0027]
[0028]Specifically, the following steps:
[0029](1) Take 1.37 g (137 g / mol, 0.01 mol) 4-amino phenylethyl alcohol in a round bottom flask, add 10 ml of water, ultrasonic stirring, complete hydrochloric acid (4.17 ml, 12 mol / L), cool down To 0-5 ° C, sodium nitrite (1.5 times, 69 g / mol, 1.035 g) was added, and a certain amount of urea was added, and a quantitative urea was added, and the extra sodium nitrite was removed.
[0030](2) Weigh 3.08 g coupling sub-H (structural formula:The coupling agent solution is obtained in 20 ml of ethanol to cool down to 1-5 ° C. Keeping step (1) The reaction system temperature is added to 0-5 ° C, and the coupling sub-solution is added dropwise to the sodium carbonate. After the reaction is complete, the post-treatment is completely frozen crystal, add water, and use acetate with petroleum ether mixed with petroleum ether. Extraction of chromopho...
Embodiment 2
[0036]This embodiment provides a multi-chromorine pyrazolinone coupling dye B (i.e., compound b), structural formulaThe synthesis route is the same as in Example 1, and the difference is that the number of carbon atoms in the alkyl group of the coupling subsequence in this experiment is more than one carbon atom in Example 1, and the structural formula of the coupling sub:
[0037]The spectrum data of Compound B was: 1H NMR (CDCl3): 13.634 (S, 3H): 13.634 (S, 3H): 13.634 (S, 3H): 13.634 (S, 3H), 7.26 (S, 6H), 4.583-4.570 ( M, 6H) 3.705-3.70 (m, 3H), 3.143-3.140 (m, 6H), 2.44 (S, 6H), 1.630-1.625 (m, 12H), 1.540-1.520 (m, 12h), 1.370-1.250 (M, 75H), 0.890-0.880 (M, 18H), the spectrum data certification structure is correct.
Embodiment 3
[0039]This embodiment provides a multi-chromorine pyrazolinoid coupling C (i.e., Compound C), the structural formula isThe synthesis route is the same as in Example 1, and the difference is that the number of carbon atoms in the alkyl carbon chain of the renovial alkyl carbon chain in this experiment is less than 6 in Example 1, and the structural formula of the coupling subscript is:
[0040]Analysis of the nuclear magnetic spectrum, the spectrum data of Compound C was: 1H NMR (CDCl3): 13.794 (S, 3H) 8.739 (S, 3H), 7.398-7.236 (M, 12H), 4.663-4.570 (m, 6H) 3.835-3.785 (m, 3H), 3.343-3.340 (m, 6H), 1.655 (M, 6H), 1.499-1.480 (m, 12H), 1.444 (M, 6H), 1.380-1.244 (m, 9h), 0.890-0.880 (M, 18H), the spectrum data proves the structure correctly.
[0041]The obtained dye light stability is good, and has a very high solubility, and the ink can be prepared in the organic solvent. In particular, in a non-polar organic solvent, such as n-decane, n-dodexecane, n-4alkane, n-hexadecane, fluorine-conta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com