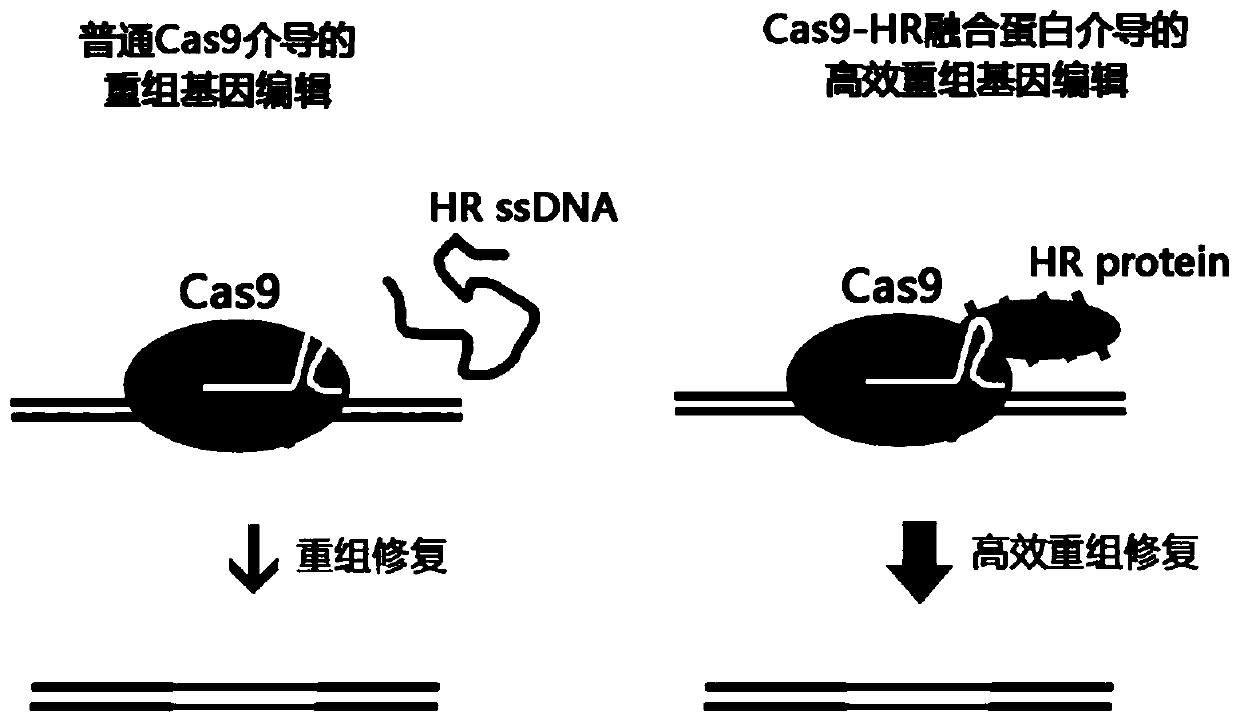
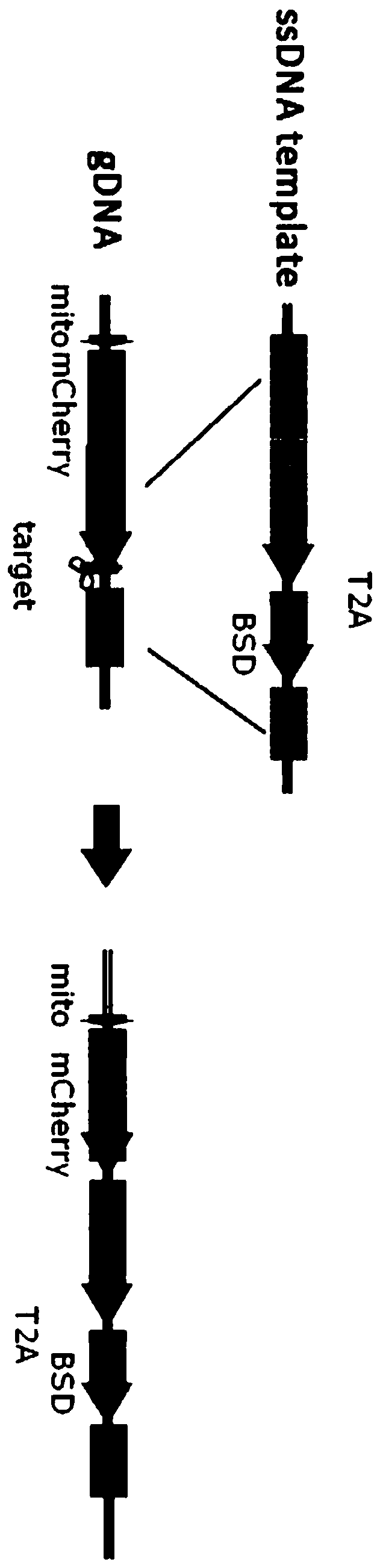
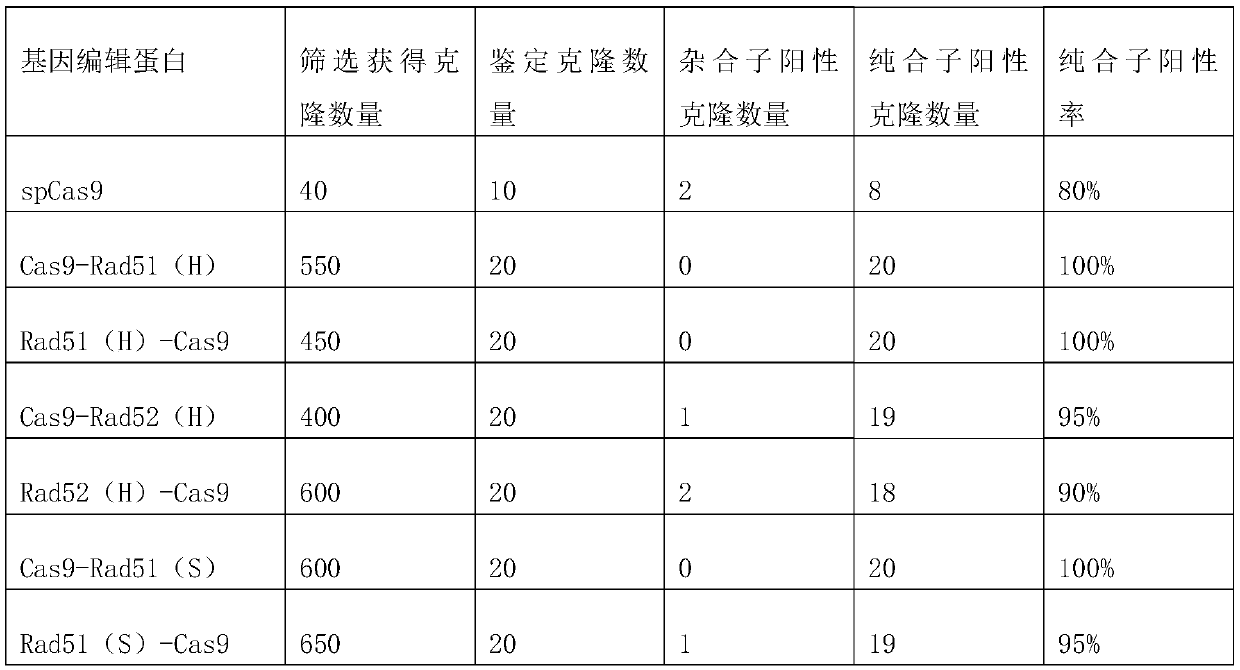
Fusion protein and design method thereof
A fusion protein and protein technology, applied in the biological field, can solve the problems of low efficiency of recombinant gene editing and less recombination repair, and achieve the effect of improving the efficiency of recombination repair
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Fusion protein 1: design method of spCas9-Rad51(H) and expression and purification of spCas9-Rad51(H) protein with E. coli expression host.
[0026] Fusion protein 1: design method of spCas9-Rad51(H).
[0027] 1. The protein functional domain with specific DNA cutting function selects spCas9 protein;
[0028] 2. Human Rad51 protein is selected as the recombinant auxiliary protein functional domain with DNA binding function;
[0029] 3. The two are connected with polypeptide GGGGSGGSGGSGGGS;
[0030] 4. The protein structure is spCas9-connecting peptide-Rad51(H).
[0031] The method of fusion expressing spCas9-Rad51(H) using Escherichia coli prokaryotic expression system, the realization steps are as follows:
[0032] 1. Connect the artificially synthesized spCas9-Rad51(H) coding sequence to the prokaryotic expression vector pET-30a to obtain the expression vector pET-spCas9-Rad51(H);
[0033] 2. Transform Escherichia coli expression strain BL21(DE3), the transformat...
Embodiment 2
[0048] Fusion protein 2: the design method of spCas9-Rad52(H), the specific implementation steps are as follows:
[0049] 1. The protein functional domain with specific DNA cutting function selects spCas9 protein;
[0050] 2. Human Rad52 protein is selected as the recombinant auxiliary protein functional domain with DNA binding function;
[0051] 3. The two are connected with polypeptide GGGGSGGSGGSGGGS;
[0052] 4. The protein structure is spCas9-connecting peptide-Rad52(H).
[0053] The expression and purification methods of spCas9-Rad52(H) protein are the same as in Example 1.
Embodiment 3
[0055] Fusion protein 3: The design method of Rad51(H)-spCas9, the specific implementation steps are as follows:
[0056] 1. The protein functional domain with specific DNA cutting function selects spCas9 protein;
[0057] 2. Human Rad51 protein is selected as the recombinant auxiliary protein functional domain with DNA binding function;
[0058] 3. The two are connected with polypeptide GGGGSGGSGGSGGGS;
[0059] 4. The protein structure is Rad51(H)-linked peptide-spCas9.
[0060] The expression and purification methods of Rad51(H)-spCas9 protein are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com