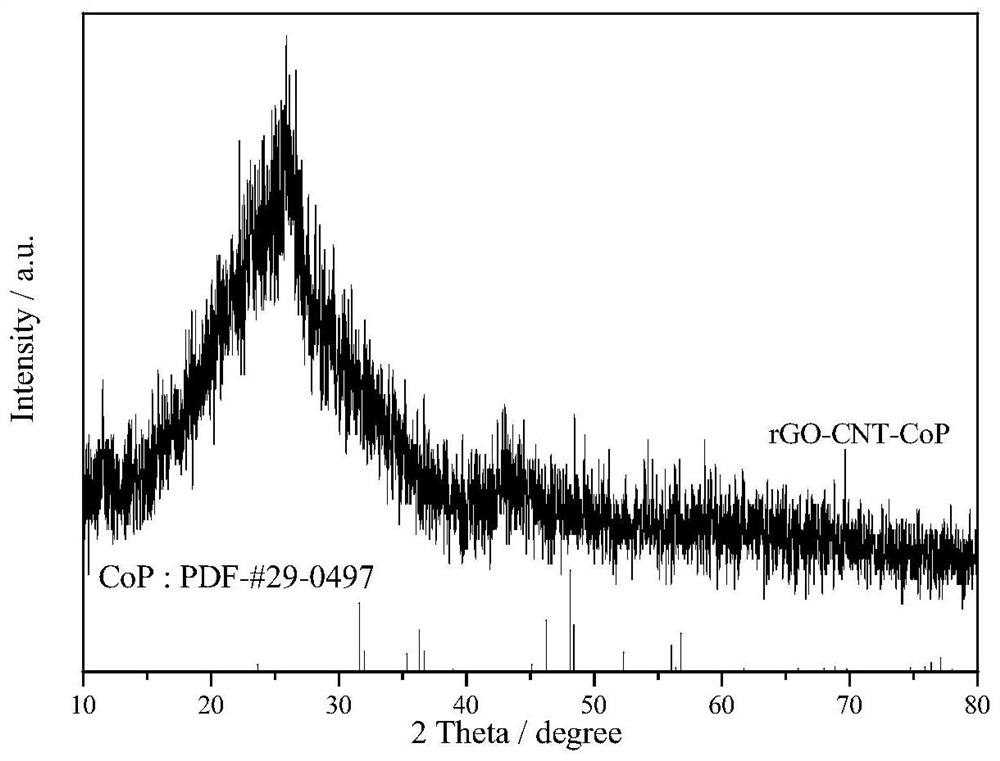
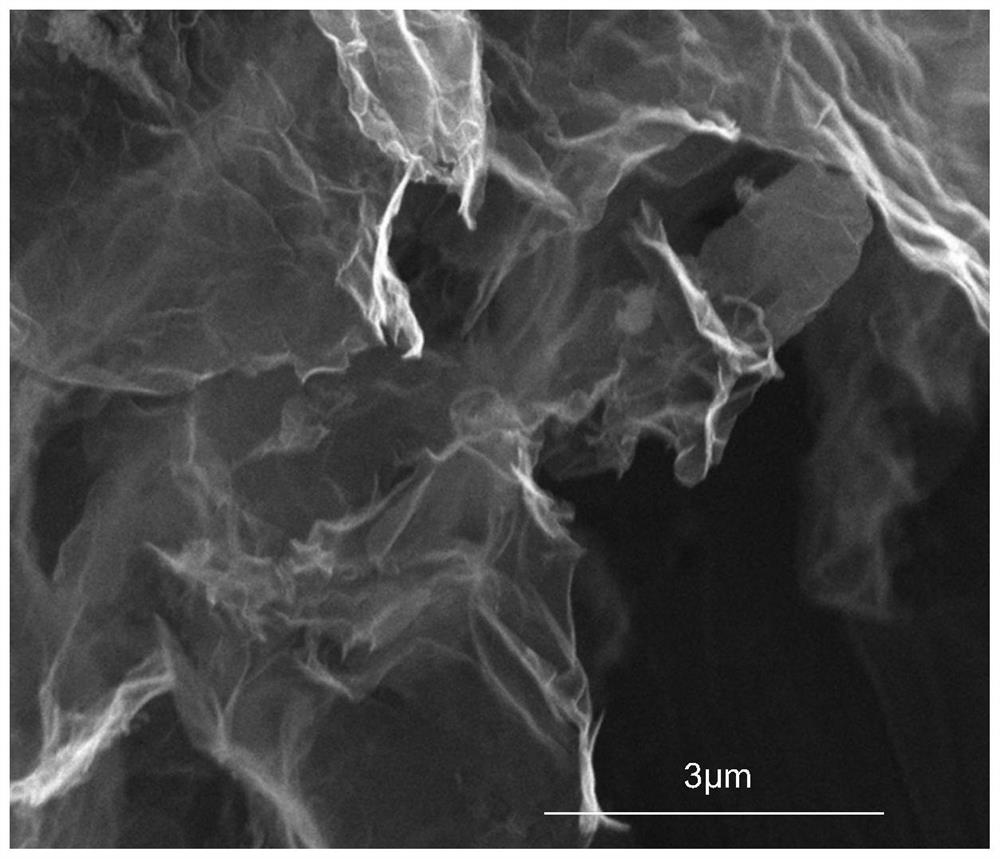
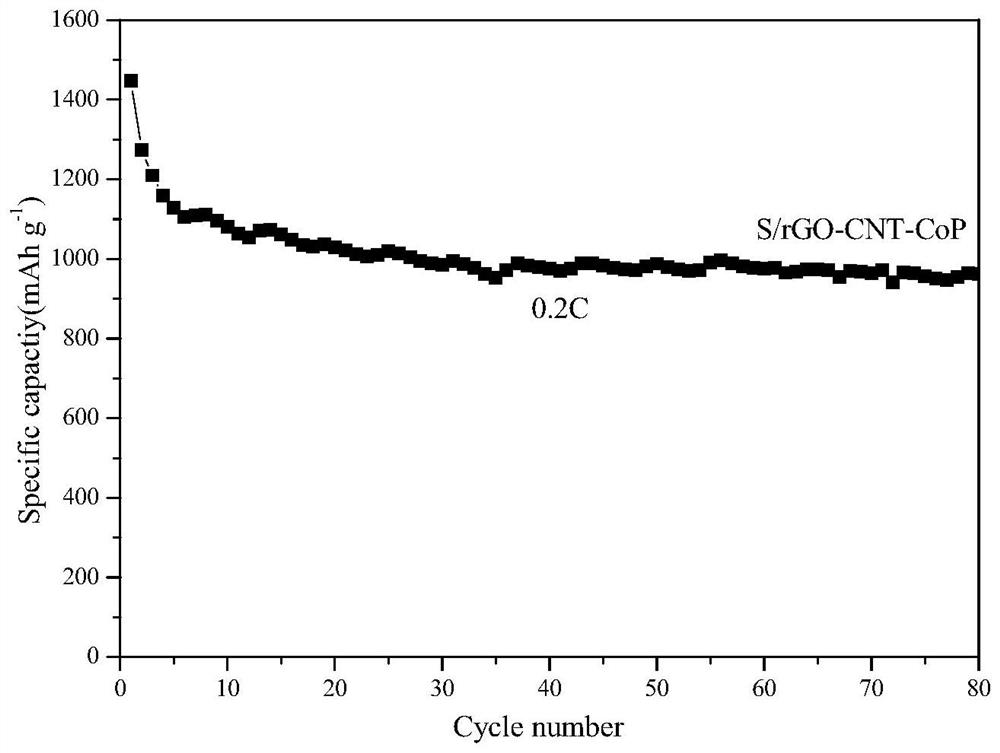
A kind of amorphous cobalt phosphide/nano carbon composite material, preparation method and application thereof
A nano-carbon material and composite material technology, applied in the field of new energy materials, can solve the problems of limited improvement of catalytic conversion kinetics, weak adsorption capacity of soluble lithium polysulfide, and easy agglomeration of crystalline CoP, so as to promote electrochemical conversion, The effect of improving catalytic kinetics and excellent electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] (1) Dissolve 49.6mg of cobalt chloride hexahydrate in a mixed solution comprising 20mL of ethanol and 20mL of water, disperse 60mg of graphene oxide and 30mg of multi-walled carbon nanotubes in the above solution, and dissolve 0.6mL of concentrated ammonia water (the ammonia content is 28wt%) was added to the above solution, stirred at room temperature for 3 hours and mixed evenly, and then ultrasonically dispersed for 30 minutes to obtain dispersion I;
[0044] (2) Heat and stir the dispersion I in an oil bath at 100° C. for 6 hours after sealing to obtain the dispersion II;
[0045] (3) Pour the dispersion II into a sealed high-temperature and high-pressure hydrothermal reaction kettle for hydrothermal reaction. The hydrothermal temperature is 200°C, and the hydrothermal time is 20h. Then, it is naturally cooled to room temperature and centrifuged to obtain the solid After washing with water and ethanol, the precursor powder was obtained after freeze-drying at -40°C f...
Embodiment 2
[0056] (1) 76.8 mg of cobalt nitrate hexahydrate is dissolved in a mixed solution comprising 30 mL of ethanol and 10 mL of water, 80 mg of graphene oxide and 10 mg of multi-walled carbon nanotubes are dispersed in the solution, and 1.2 mL of concentrated ammonia ( The ammonia content is 28wt%) was added to the above solution, stirred at room temperature for 6 hours and mixed evenly, and then ultrasonically dispersed for 50 minutes to obtain dispersion I;
[0057] (2) Heat and stir the dispersion I in an oil bath at 90°C for 12 hours after sealing to obtain the dispersion II;
[0058] (3) Pour the dispersion II into a sealed high-temperature and high-pressure hydrothermal reaction kettle for hydrothermal reaction. The hydrothermal temperature is 220°C, and the hydrothermal time is 14h. Then, it is naturally cooled to room temperature and centrifuged to obtain a solid After washing with water and ethanol, the precursor powder was obtained after freeze-drying at -40°C for 12 hour...
Embodiment 3
[0069] (1) 93.6 mg of cobalt acetate tetrahydrate is dissolved in a mixed solution comprising 10 mL of ethanol and 30 mL of water, 60 mg of graphene oxide and 5 mg of multi-walled carbon nanotubes are dispersed in the solution, and 1.0 mL of concentrated ammonia ( The ammonia content is 28wt%) was added to the above solution, stirred at room temperature for 6 hours and mixed evenly, and then ultrasonically dispersed for 30 minutes to obtain dispersion I;
[0070] (2) After sealing the dispersion I, heat and stir in an oil bath at 120°C for 8 hours to obtain the dispersion II;
[0071] (3) Pour the dispersion II into a sealed high-temperature and high-pressure hydrothermal reaction kettle for hydrothermal reaction. The hydrothermal temperature is 150°C, and the hydrothermal time is 24h. Then, it is naturally cooled to room temperature and centrifuged to obtain a solid After washing with water and ethanol, the precursor powder was obtained after freeze-drying at -50°C for 24 hou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com