Composition for preventing and treating hyperuricemia and gout and application thereof
A composition, high uric acid technology, applied in the application, drug combination, food science and other directions, can solve the problems of inability to store for a long time, low stability, poor absorption and metabolism, etc., to prevent excessive formation, improve the obstruction of discharge channels, The effect of lowering uric acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0111] The preparation method of the composition of the present invention comprises the following steps: uniformly mixing each component according to the weight parts and weight ratios, and sterilizing to obtain the finished product.
[0112] The composition of the present invention is any one of tablets, powders, granules, and capsules, and is not specifically limited herein, and can be determined according to actual production needs and consumer demands.
[0113] The second aspect of the present invention provides an application of the composition for preventing and treating hyperuricemia and gout in the preparation of products and / or product additives for reducing uric acid and enhancing body immunity.
[0114] In one embodiment, the product includes one or a combination of solid beverages, liquid beverages, dairy products, seasoning powders, cold drinks, health nutrition products and health care products.
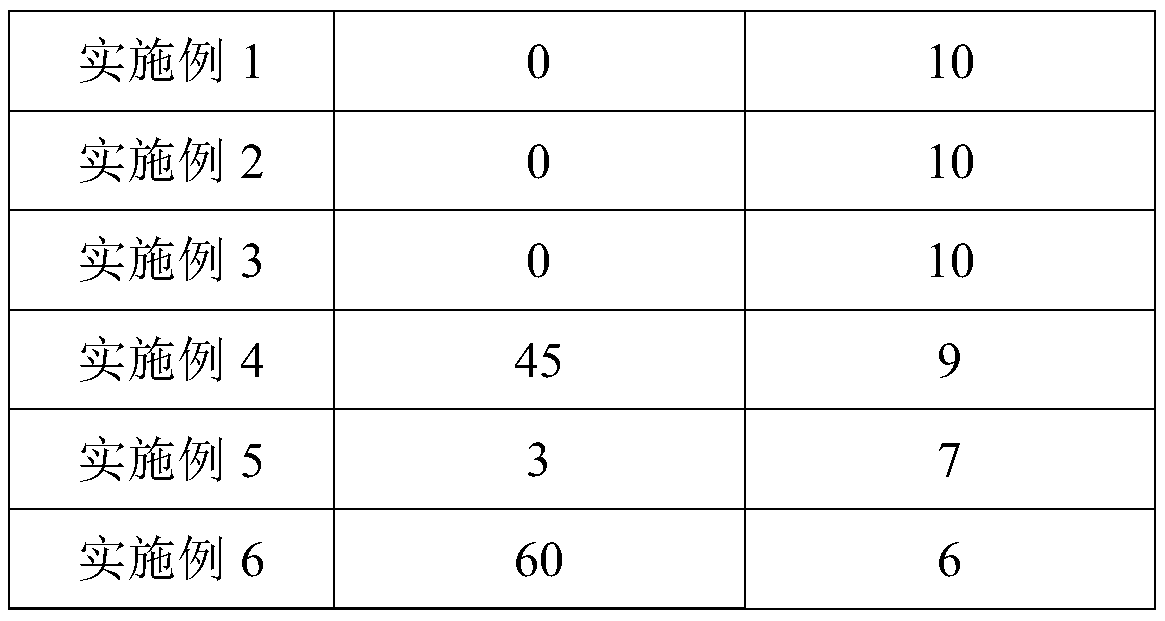
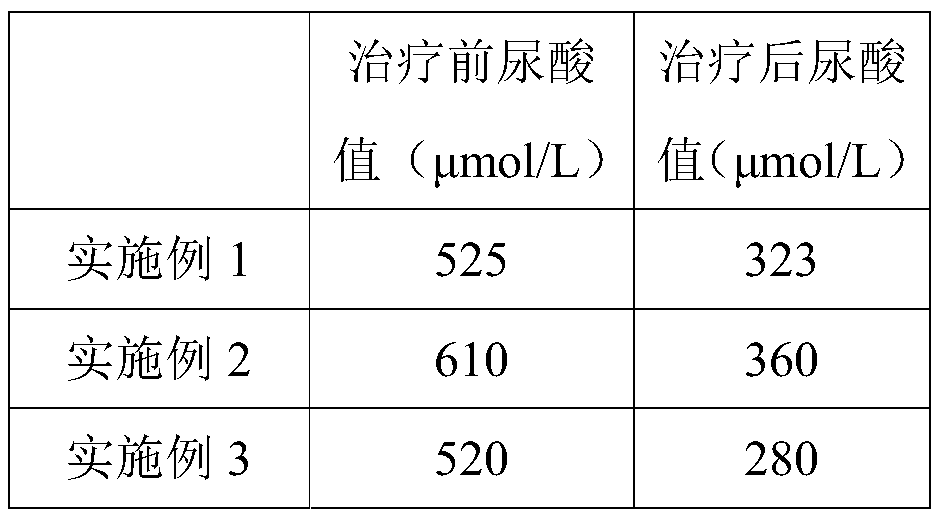
Embodiment 1
[0118] Embodiment 1 of the present invention provides a kind of composition that is used for preventing and treating high uric acid and gout, and by weight, component comprises 120 parts of tuna peptide powders, 35 parts of aminobutyric acid, 18 parts of chitosan oligosaccharides, 20 parts of fruit powders , 100 parts of plant powder, 1.4 parts of vitamins.
[0119]The molecular weight of the chitosan oligosaccharide is 1000Da, purchased from Qingdao Bozhi Huili Biotechnology Co., Ltd., the model is dp3-7.
[0120] The tuna peptide powder includes low-molecular-weight tuna peptide powder and high-molecular-weight tuna peptide powder; the weight ratio of the low-molecular-weight tuna peptide powder to the high-molecular-weight tuna peptide powder is 1:0.6; the molecular weight of the low-molecular-weight tuna peptide powder is 200 ~400Da; the molecular weight of the high molecular weight tuna peptide powder is 500~1000Da.
[0121] The aminobutyric acid is gamma-aminobutyric ac...
Embodiment 2
[0140] Example 2 of the present invention provides a composition for preventing and treating high uric acid and gout. Aminobutyric acid, 34 parts of chitosan oligosaccharides, 35 parts of fruit powder, 161 parts of plant powder, 2.5 parts of vitamins; the weight ratio of the low molecular weight tuna peptide powder and high molecular weight tuna peptide powder is 1:0.7; the multivitamin and The weight ratio of vitamin C is 1:8; in parts by weight, the plant powder includes 41 parts of inulin, 3 parts of lotus leaf powder, 34 parts of poria powder, 18 parts of barley powder, 7 parts of wolfberry powder, and 7 parts of sealwort powder , 2 parts of cassia seed powder, 23 parts of lemon powder, 20 parts of sweet orange powder, 6 parts of passion fruit powder and 28 parts of acerola powder; the weight ratio of the short-chain inulin and long-chain inulin is 0.4:1; by weight In terms of parts, the flavor enhancer includes 2.3 parts of halogenated sucrose derivatives, 39 parts of oli...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com