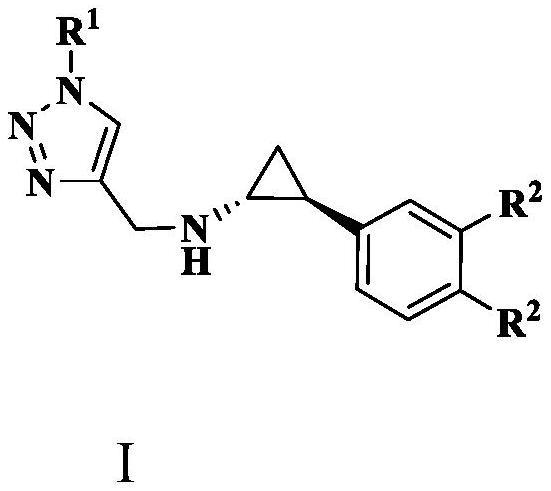
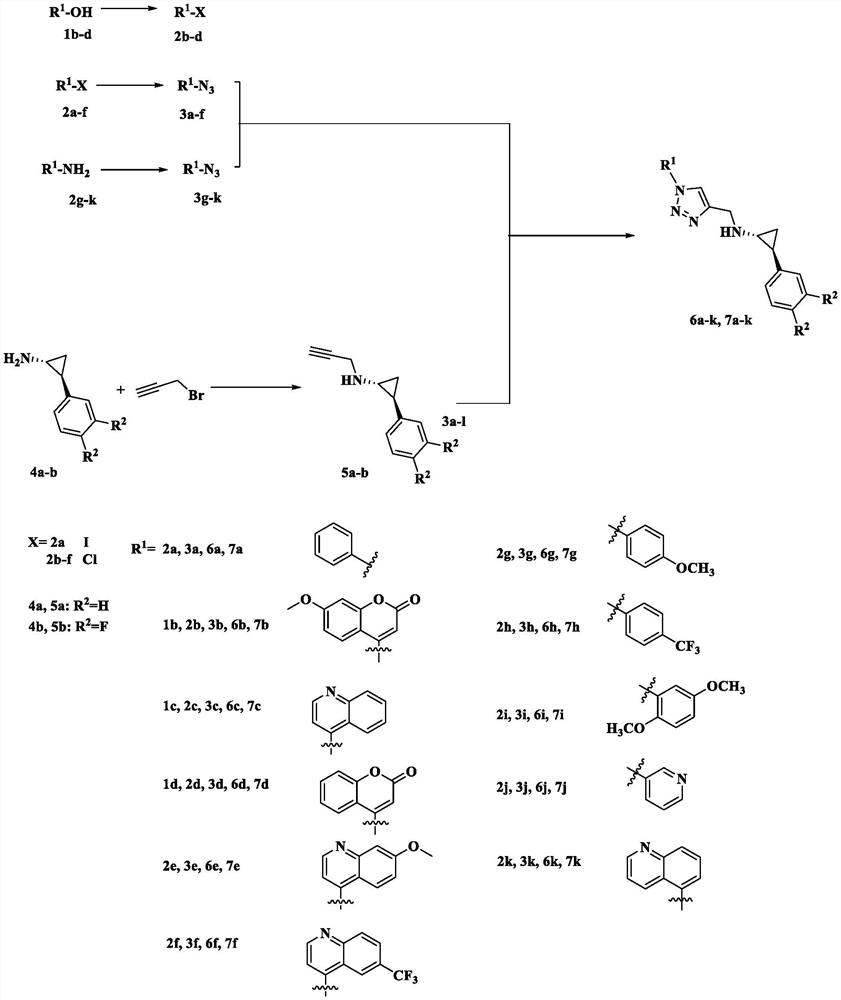
Containing aromatic ring/aromatic heterocycle-triazole-methylene-tcp derivative and its preparation method and application
A technology of aromatic heterocycles and triazoles, which is applied in the field of medicinal chemistry, can solve the problem that LSD1 has no inhibitory effect, and achieve the effects of rich variety, good application prospects, and convenience for mass production and commercial application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation shown in general formula Ⅰ: wherein R 1 for R 2 is a hydrogen atom, that is, compound 6b(1R,2S)-2-phenyl-N-((1-(7-methoxycoumarin-4-yl)-1H-1,2,3-triazole -4-yl) methyl) cyclopropyl-1-amine, the process is as follows:
[0046] (1) Synthesis of compound 5a (1R, 2S)-2-phenyl-N-propargylcyclopropyl-1-amine): take compound 4a trans-phenylcyclopropylamine free state (2.0g , 15mmol) in a 100mL Shrek bottle, add triethylamine (2.07g, 15mmol), acetonitrile 45mL, propyne bromide (1.17mL, 15mmol), stir at room temperature overnight, concentrate after the reaction is completed, dichloromethane (70mL×3 ), dried over sodium sulfate, and separated by neutral alumina column chromatography to obtain pale yellow liquid 5a (1.54 g, 9 mmol), with a yield of 55%. 1 H NMR (600MHz, Chloroform-d) δ7.25(t, J=7.4Hz, 2H), 7.15(t, J=7.3Hz, 1H), 7.06(d, J=7.7Hz, 2H), 3.49(s , 2H), 2.60(m, 1H), 2.22(s, 1H), 1.97(m, 1H), 1.91(s, 1H), 1.09(m, 1H), 0.98(m, 1H). 13 C NMR (151 MHz, Chl...
Embodiment 2
[0051] Preparation shown in general formula Ⅰ: wherein R 1 for R 2 is a hydrogen atom, that is, compound 6e(1R,2S)-2-phenyl-N-((1-(7-methoxyquinolin-4-yl)-1H-1,2,3-triazole- 4-yl) methyl) cyclopropyl-1-amine, the process is as follows:
[0052] (1) The synthesis process of compound 5a is the same as in Example 1.
[0053] (2) Synthesis of compound 3e (4-azido-7-methoxyquinoline): Take compound 2e (0.97g, 5mmol) in a 50mL round bottom flask, add 15mL of N,N-dimethylformamide, Sodium azide (488 mg, 7.5 mmol) was stirred at room temperature for 8 h. After the reaction was monitored by TLC, it was extracted with ethyl acetate (70 mL×3), dried over sodium sulfate, and concentrated to obtain the crude product 3e.
[0054] (3) Compound 6e(1R, 2S)-2-phenyl-N-((1-(7-methoxyquinolin-4-yl)-1H-1,2,3-triazole-4- Synthesis of base) methyl) cyclopropyl-1-amine: Take compound 5a (171mg, 1mmol) in a 25mL Shrek tube, add 3e (200mg, 1mmol), copper sulfate pentahydrate (12.5mg, 0.05mmol), ...
Embodiment 3
[0056] Preparation shown in general formula Ⅰ: wherein R 1 for R 2 is a fluorine atom, that is, compound 7b(1R,2S)-2-(3,4-difluorophenyl)-N-((1-(7-methoxycoumarin-4-yl)-1H-1 , 2,3-triazol-4-yl) methyl) cyclopropyl-1-amine, the preparation process is as follows:
[0057] (1) Synthesis of compound 5b(1R, 2S)-2-(3,4-difluorophenyl)-N-(propargyl)cyclopropyl-1-amine: take compound 4b( 1R, 2S)-3,4-difluorophenylcyclopropylamine free state (2.5g, 15mmol) in a 100mL Shrek bottle, add potassium carbonate (2.07g, 15mmol), acetonitrile 45mL, propyne bromide (1.17mL, 15mmol ), stirred at room temperature overnight, after the reaction was completed, concentrated, extracted with dichloromethane (70mL × 3), dried over sodium sulfate, and separated by neutral alumina column chromatography to obtain light yellow liquid 5b (1.6g, 7.7mmol), the yield 53%. 1 H NMR (600MHz, Chloroform-d) δ7.02(m, 1H), 6.84(m, 1H), 6.79(m, 1H), 3.49(d, J=2.3Hz, 2H), 2.54(m, 1H) , 2.24(t, J=2.4Hz, 1H), 1.93(m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com