Method for extracting monoterpene glycoside type effective components from nonmedicinal parts of paeonia lactiflora
A technology of medicinal parts and monoterpene glycosides is applied in the field of extraction of effective components of traditional Chinese medicine, which can solve the problems of extraction and have not yet seen effective components, and achieve the effect of reducing potential toxic components and enriching and effectively comprehensive utilization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0185] This example aims to illustrate a method for extracting active ingredients from the non-medicinal parts of Radix Paeoniae Alba, the specific steps are as follows:
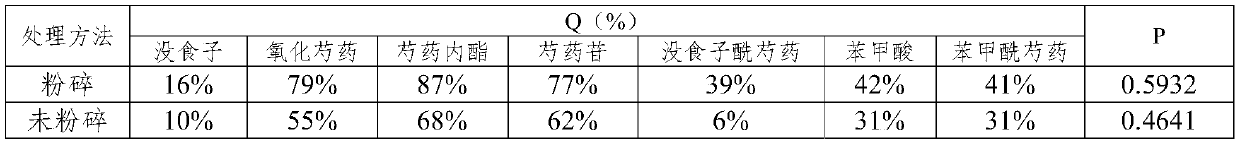
[0186] (1) Dry and pulverize the non-medicinal parts of Radix Paeoniae Alba, pass through a 10-mesh sieve to obtain a granular sample;
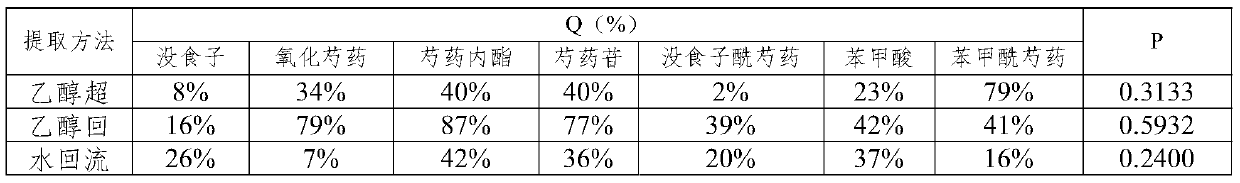
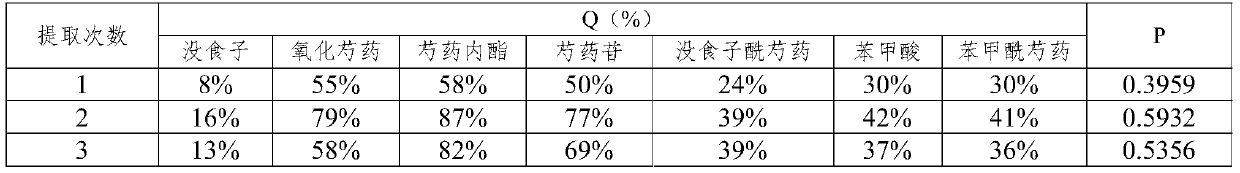
[0187] (2) Using 12 times the volume of 50% ethanol, heat and reflux extraction twice, each extraction time is 80min, combine the extracts, filter and concentrate to obtain a crude extract with a concentration of 0.25-0.5g dry raw materials / mL;
[0188](3) Adopt a chromatographic column with a column diameter-to-height ratio of 1:4, use D-101 macroporous resin as a filler, load 3BV of the crude extract at a loading speed of 0.5mL / min, and then use 2 times volume of water and 4 times the volume of 50% ethanol to elute the macroporous resin chromatography column at an elution rate of 0.75mL / min, collect the eluate from the elution site of 50% ethanol, concentrate, and dry to ...
Embodiment 2
[0190] The difference between this example and Example 1 is that 12 times the volume of 50% ethanol in step (2) is replaced by 10 times the volume of 70% ethanol. Precisely weigh the refined extract dried to constant weight, and carry out content determination according to the above method, then the content of each component is as follows: paeoniflorin 38.9%, paeoniflorin 17.6%, benzoyl paeoniflorin 1.85%, galloyl paeoniflorin Glycoside 1.45%, oxidized paeoniflorin 0.34%, gallic acid 0.045%, benzoic acid 1.5%, and the total content of monoterpene glycosides in the effective part is 60.14%. The yield of purified products in this process is 7.2%, so the transfer rate of monoterpene glycosides is 75.5%, the transfer rate of gallic acid is 0.40%, and the transfer rate of benzoic acid is 46.2%.
Embodiment 3
[0192] The difference between this example and Example 1 is that in step (3), the macroporous resin chromatography column is eluted with 2 times the volume of water and 4 times the volume of 50% ethanol at an elution rate of 1 mL / min. Precisely weigh the refined extract dried to constant weight, and carry out content determination according to the above method, then the content of each component is as follows: paeoniflorin 38.64%, paeoniflorin 17.5%, benzoyl paeoniflorin 1.85%, galloyl paeoniflorin Glycoside 1.93%, oxidized paeoniflorin 0.33%, gallic acid 0.033%, benzoic acid 1.1%, and the total content of monoterpene glycosides in the effective part is 60.25%. The yield of purified products in this process is 7.2%, so the transfer rate of monoterpene glycosides is 74.7%, the transfer rate of gallic acid is 0.29%, and the transfer rate of benzoic acid is 33.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com