Cyclic endomorphin-1 analog with high enzymolysis stability and analgesic activity and application thereof
An endomorphin and analog technology, which can be applied to medical preparations containing active ingredients, cyclic peptide components, peptides, etc., can solve the problems of short analgesic effect time and poor enzymatic stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
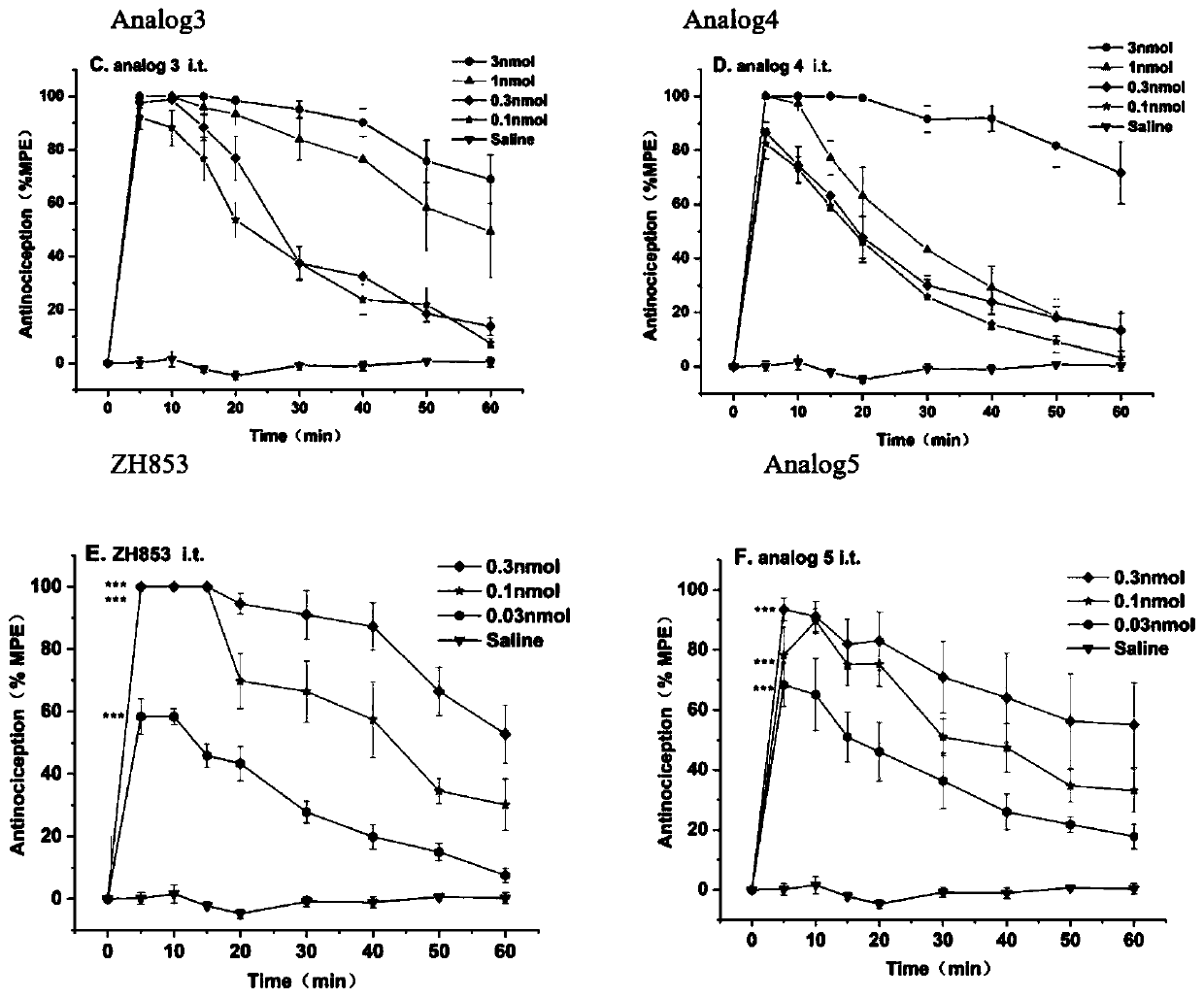
[0074] Embodiment 1: the synthesis of Analog3
[0075] (1) Solid-phase synthesis of linear peptides: put 0.3 mmol of Rink amide MBHA resin in a solid-phase synthesizer, add 10 mL of redistilled DCM to swell and activate, and use DMF to elute the DCM, and use it when the resin is in good condition. DMF eluting indene detection reagent; sequentially add 0.9 mmol of Fmoc-protected cyclic endomorphin-1 analog 1-position amino acid, HOBt, HBTU, and 0.3 mL of DIEA in DMF solution, and react for 40 min under the protection of inert gas; After elution of unreacted drugs and reagents in the DMF reaction system, check whether the amino acid is connected to the resin; after that, the connection of the 2-6 amino acid is the same as the above process, and the linear peptide is obtained;
[0076] (2) Cyclization of linear peptides: transfer the resin connected with linear peptides in step (1) to a round bottom flask, add CCl with a volume ratio of 37:2:1 to 1g of resin corresponding to 15mL...
Embodiment 2
[0080] Embodiment 2: the synthesis of Analog4
[0081] (1) Solid-phase synthesis of linear peptides: put 0.3mmol Rink amide MBHA resin in a solid-phase synthesizer, add 12mL redistilled DCM to swell and activate, after DMF elutes DCM, if the resin is in good condition, wash it with DMF. Indene detection reagent; sequentially add 0.6mmol of Fmoc-protected 1-position amino acid of Analog4, HOBt, HBTU, and 0.2mL of DIEA in DMF solution, and react for 50min under the protection of inert gas; DMF elutes unreacted drugs in the reaction system Check whether the amino acid is connected to the resin after indene with the reagent; after that, the insertion of the 2-6 amino acid is the same as the above process to obtain a linear peptide;
[0082] (2) Cyclization of linear peptides: transfer the resin connected with linear peptides in step (1) to a round bottom flask, add CCl with a volume ratio of 37:2:1 to 1g of resin corresponding to 15mL of solvent under magnetic stirring 3 , HAC an...
Embodiment 3
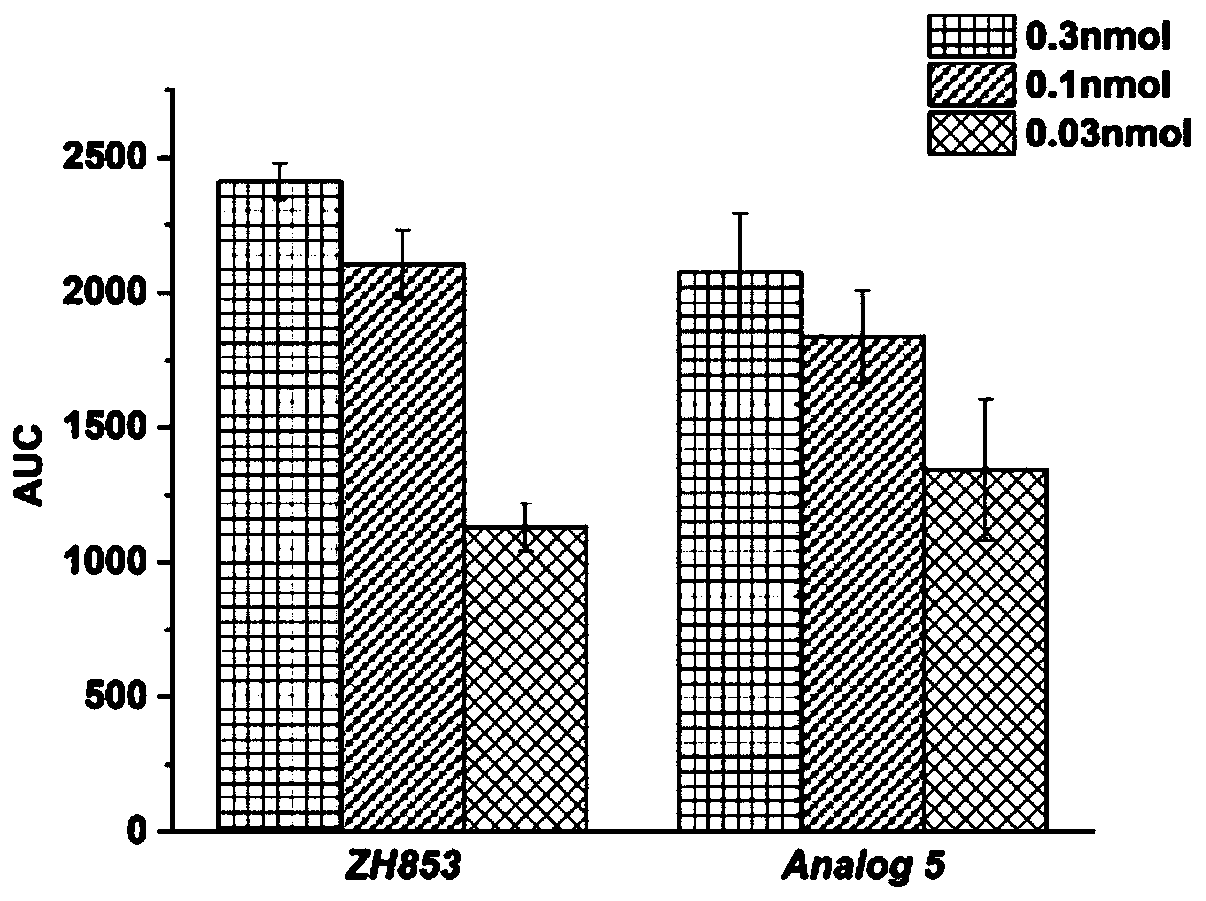
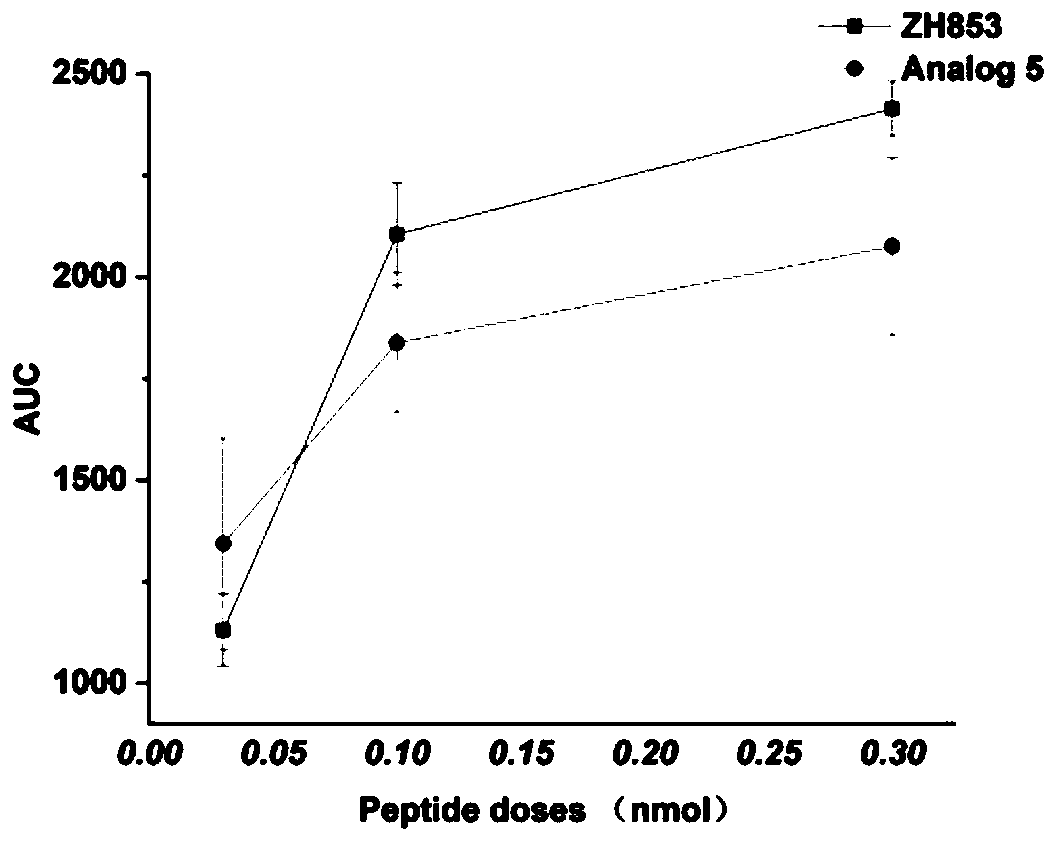
[0086] Embodiment 3: the synthesis of Analog5
[0087](1) Solid-phase synthesis of linear peptides: put 0.3mmol of Rink amide MBHA resin in a solid-phase synthesizer, add 15mL of redistilled DCM for swelling and activation, and use DMF to elute DCM. If the resin is in good condition, use DMF elutes the indene detection reagent; sequentially add 0.45mmol of Fmoc-protected 1-position amino acid of cyclic Analog5, HOBt, HBTU, and 0.1mL of DIEA in DMF, react for 60min under the protection of inert gas; DMF elution reaction system After the unreacted drugs and reagents, check whether the amino acid is connected to the resin; after that, the connection of the 2-6 amino acid is the same as the above process, and the linear peptide is obtained;
[0088] (2) Cyclization of linear peptides: transfer the resin connected with linear peptides in step (1) to a round bottom flask, add CCl with a volume ratio of 37:2:1 to 1g of resin corresponding to 15mL of solvent under magnetic stirring 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com