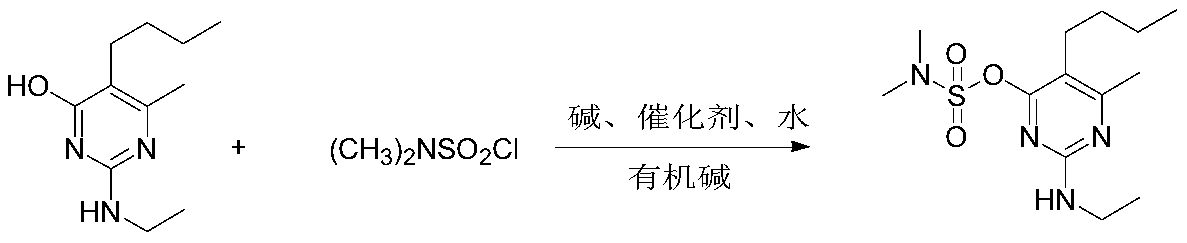
Synthetic method of bupirimate
A technology of pyrimethamine sulfonate and synthesis method, applied in directions such as organic chemistry, can solve problems such as complicated operation, and achieve the effect of reducing operation steps and production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 20.9g of pyrimol, 13.9g of potassium carbonate, 180ml of toluene, 1.8ml of water, and 0.01% of 18-crown-6 into the reaction flask in sequence, raise the temperature to 70°C under stirring, and slowly add 14.4g of N,N-dimethyl Sulfonyl chloride, after dropping, the temperature was raised to reflux for 2 hours, and triethylamine was added dropwise to control the pH of the system to 9 when 50% of the remaining raw materials were detected, and the reaction was continued for 4 hours until the end. Then the system was cooled to 55°C, washed with water to adjust the system to neutral, the water phase was separated, and the solvent was removed to obtain an oily substance. The solid was precipitated at a low temperature of 10°C, dried and weighed 29.4g, which was pyrimetholsulfonate .
[0031] Yield 93%, content 98%.
[0032] 1 HNMR(500MHz,DMSO):δ0.9(t,3H),1.13(t,3H),1.2~1.5(m,4H),2.3(s,3H),2.4(t,3H),2.9(s, 6H), 3.2(m,2H), 7.16(s,1H); IR(KBr,cm -1 ):3420,2960,2875,2858,1...
Embodiment 2
[0035] Add 20.9g of pyrimol, 15.9g of sodium carbonate, 180ml of toluene, 0.18ml of water, and 0.1% tetramethylammonium bromide into the reaction flask in sequence, raise the temperature to 70°C under stirring, and slowly add 17.3g of N,N-di Methanesulfonyl chloride, after dropping, the temperature was raised to reflux for 2 hours, and an equal amount of triethylamine was added to control the pH of the system to 10 when the remaining 15% of raw materials were detected, and the reaction was continued for 3 hours until the end. Then the system was cooled to 60°C, washed with water to adjust the system to neutral, the water phase was separated, and the oil was obtained after removing the solvent. The solid was precipitated at a low temperature to 0°C, dried and weighed 29.4g, which was pyrimetholsulfonate , yield 93%, content 97%.
Embodiment 3
[0037] Add 20.9g of pyrimol, 10.6g of sodium carbonate, 180ml of benzene, 2ml of water, and 0.05% tetramethylammonium chloride into the reaction flask in sequence, raise the temperature to 75°C under stirring, and slowly add 14.4g of N,N-dimethyl Sulfonyl chloride, after dropping, the temperature was raised to reflux for 2 hours. When 5% of the raw material was detected, an equal amount of pyridine was added to control the pH of the system to 9, and the reaction was continued for 1 hour until the end. Then the temperature of the system was lowered to 50°C, washed with water to adjust the system to neutral, the water phase was separated, and the oil was obtained after the solvent was removed. The solid was precipitated at a low temperature of 10°C, dried and weighed 29.5g, which was pyrimetholsulfonate , yield 93.5%, content 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com