Fluorine-substituted bimolecular carbazole derivatives, preparation method and application thereof
A molecular carbazole and derivative technology, applied in the field of medicinal chemistry, can solve the problems of high toxicity and side effects, affecting the overall stability of chromosomes, and low selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
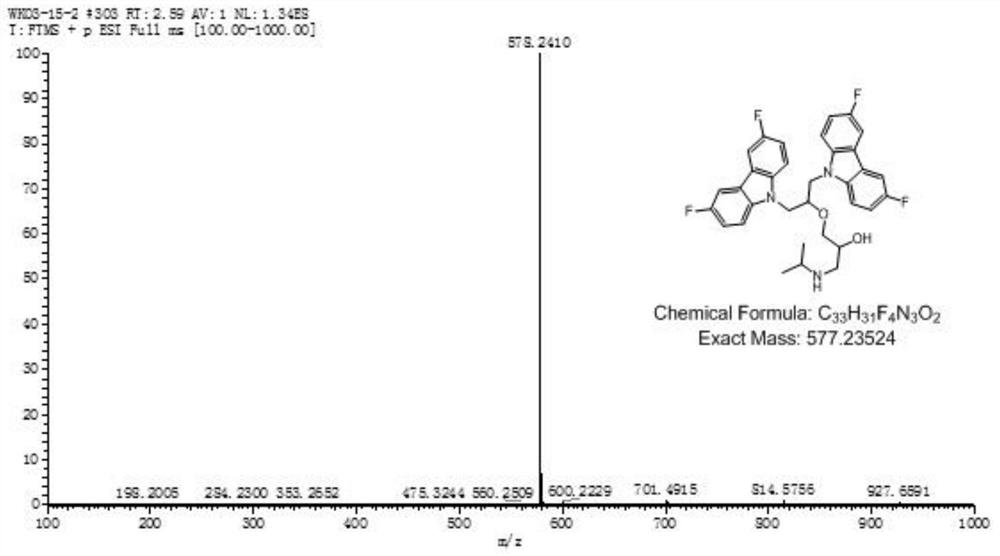
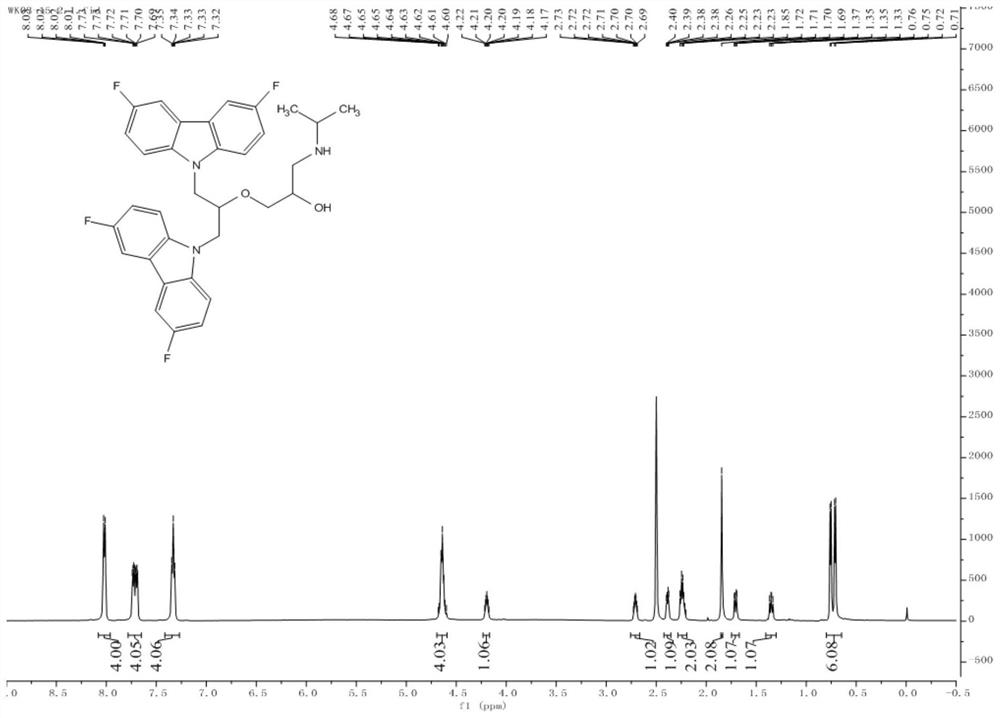
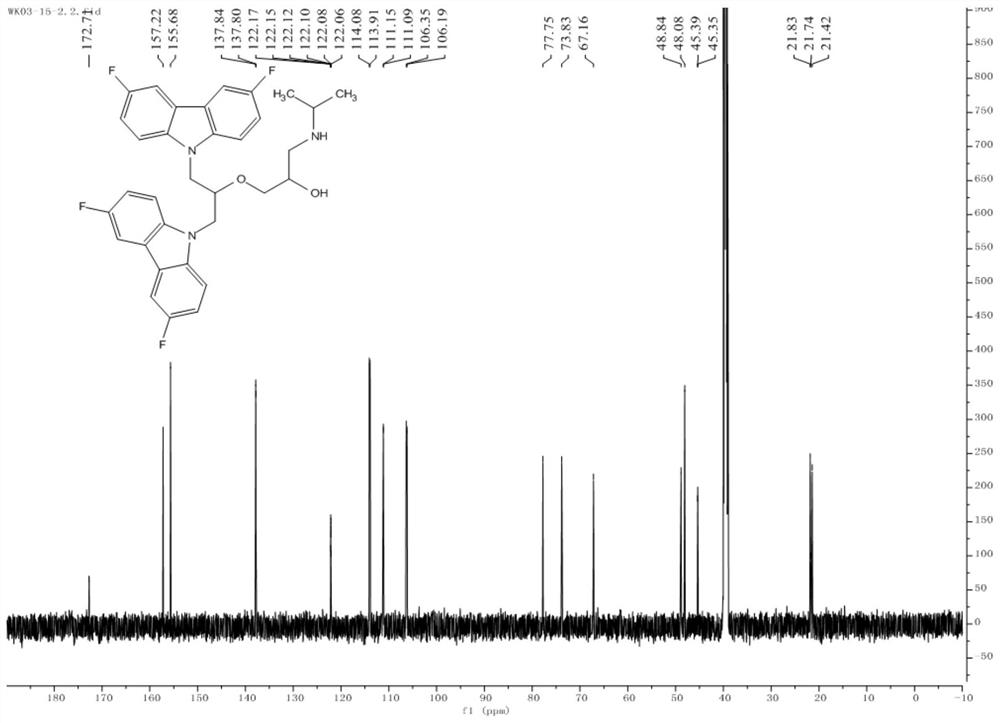
[0120] Example 1 Compound 1: 1-((1,3-bis(3,6-difluoro-9H-carbazol-9-yl)propan-2-yl)oxy)-3-(isopropylamino) -2-propanol (1-((1,3-bis(3,6-difluoro-9H-carbazol-9-yl)propan-2-yl)oxy)-3-(isopropylamino)propan-2-ol) Synthesis
[0121] It includes the following steps:
[0122] (1) Intermediate 3,6-difluoro-9-(oxiran-2-ylmethyl)-9H-carbazole (3,6-difluoro-9-(oxiran-2-ylmethyl)-9H- Synthesis of carbazole, b4):
[0123] The synthetic route is as follows:
[0124]
[0125] Concrete synthetic steps include:
[0126] Take 2.70g (13.3mmol) 3,6-difluoro-9H-carbazole (b3) to a 100mL round bottom flask, add an organic solvent (in this embodiment, the organic solvent is DMF, in other embodiments, organic The solvent can also be dissolved in about 50 mL of an aprotic solvent such as DMSO (dimethyl sulfoxide), and then 0.82 g (14.6 mmol) of the basic catalyst KOH is added, and stirred for 5 minutes in an ice bath to cool down. 2.46g (26.6mmol) epichlorohydrin was added dropwise in batche...
Embodiment 2
[0157] Example 2 Compound 2: 1-((1,3-bis(3-fluoro-9H-carbazol-9-yl)propan-2-yl)oxy)-3-(isopropylamino)-2- Synthesis of propanol (1-((1,3-bis(3-fluoro-9H-carbazol-9-yl)propan-2-yl)oxy)-3-(isopropylamino)propan-2-ol)
[0158] It includes the following steps:
[0159] (1) Intermediate 3-fluoro-9-(oxiran-2-ylmethyl)-9H-carbazole (3-fluoro-9-(oxiran-2-ylmethyl)-9H-carbazole, a4) synthesis:
[0160] The synthetic route is as follows:
[0161]
[0162] Concrete synthetic steps include:
[0163] Get 2.40g (13.0mmol) 3-fluoro-9H-carbazole (a3) to a 100mL round bottom flask, add an organic solvent (in this embodiment, the organic solvent is DMF, in other embodiments, the organic solvent can also An aprotic solvent such as DMSO) was dissolved in about 50 mL, and then 0.80 g (14.3 mmol) of the basic catalyst KOH was added, stirred for 5 minutes in an ice bath and cooled. Add 2.40 g (26.0 mmol) of epichlorohydrin dropwise in batches, and then return to room temperature and stir f...
Embodiment 3
[0195] Example 3 Compound 3: 1-((1,3-bis(3-fluoro-9H-carbazol-9-yl)propan-2-yl)oxy)-3-(tert-butylamino)-2- Synthesis of propanol (1-((1,3-bis(3-fluoro-9H-carbazol-9-yl)propan-2-yl)oxy)-3-(tert-butylamino)propan-2-ol)
[0196] Including the following steps:
[0197] Take 120.0 mg (0.2 mmol) of intermediate d4 into a 10 mL sealed tube, add 5 mL of isopropanol, add 182.5 mg (2.5 mmol) of tert-butylamine, and heat at 60°C for reaction. TLC detection (PE:EA:MeOH=5:20:3 developed) complete reaction, adding water, extraction with ethyl acetate, organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain a crude product of 0.11 g. Purified by column chromatography (EA:MeOH=20:1) to obtain 26.0 mg of a white solid with a yield of 18.84%.
[0198] Wherein, the synthesis method of intermediate d4 is the same as that in Example 2.
[0199] The synthesized compound 3 was analyzed by infrared spec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com