Macrocyclic extended porphyrin compound containing carbazole unit and preparation method thereof
A technology of porphyrin compound and carbazole unit, applied in macrocyclic extended porphyrin compound and its synthesis, extended porphyrin and its preparation field, can solve the problems such as few reports of meso-position unsubstituted extended porphyrin and the like, and achieve excellent Solubility, easy control, effect of simple purification method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
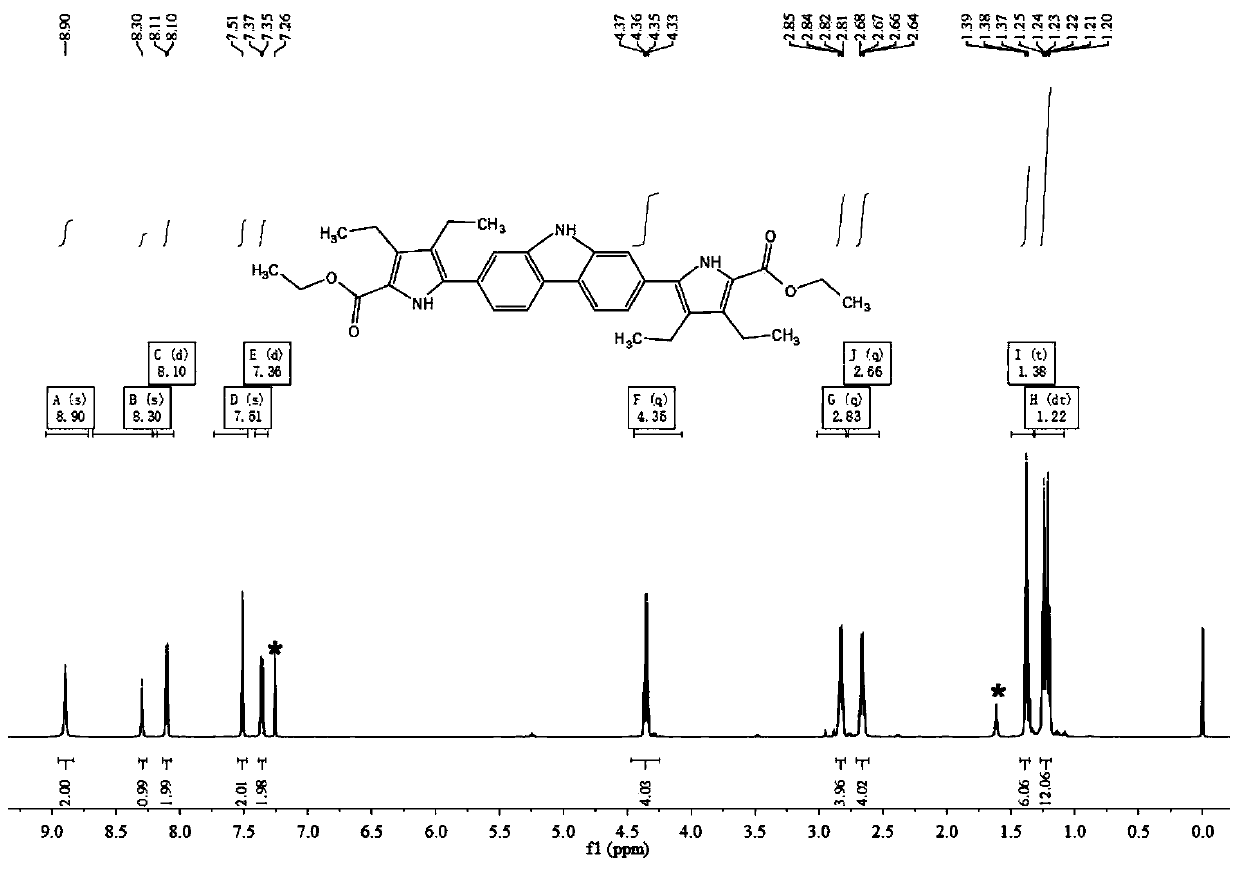
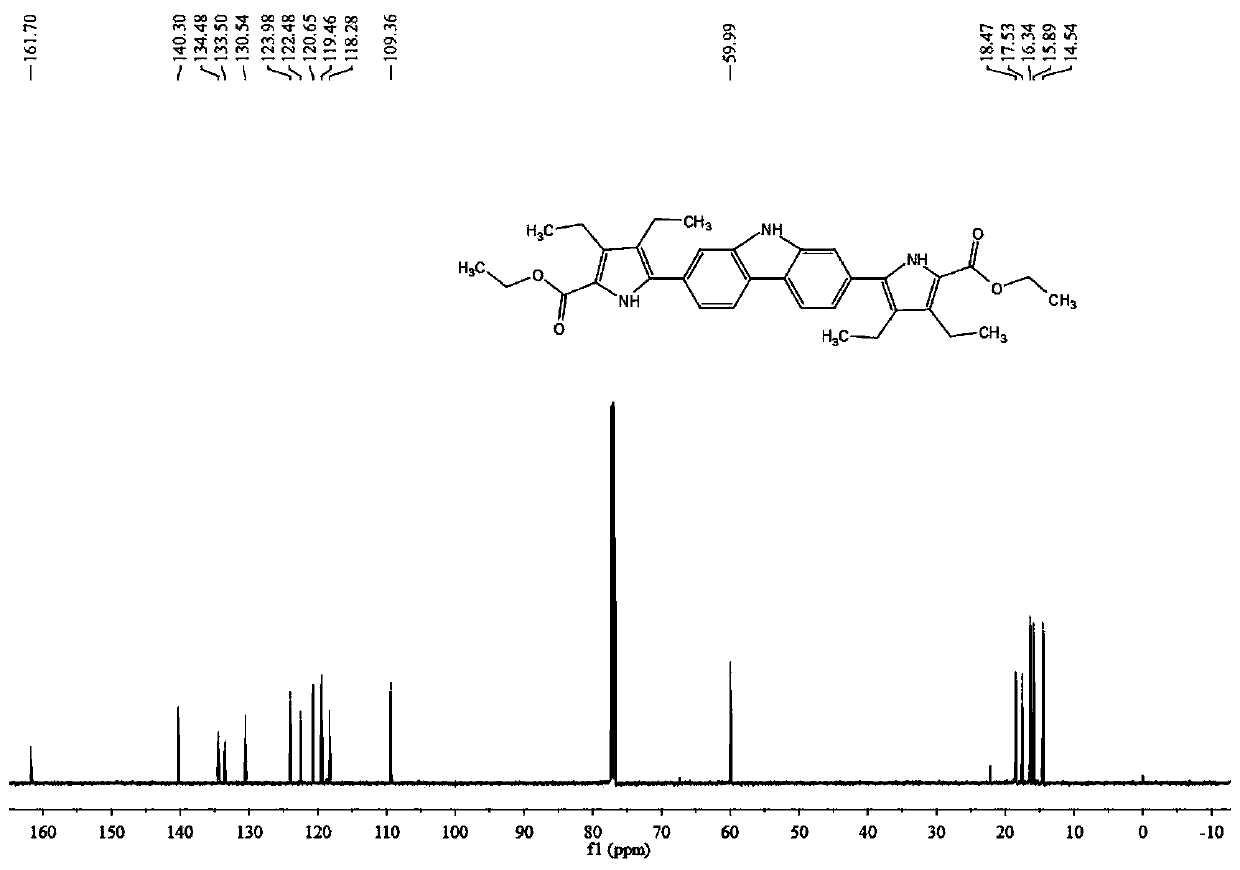
[0068] In this embodiment, a macrocyclic extended porphyrin compound containing a carbazole unit has a chemical structure of the general formula 1,
[0069]
[0070] . Its synthetic route is as follows:
[0071]
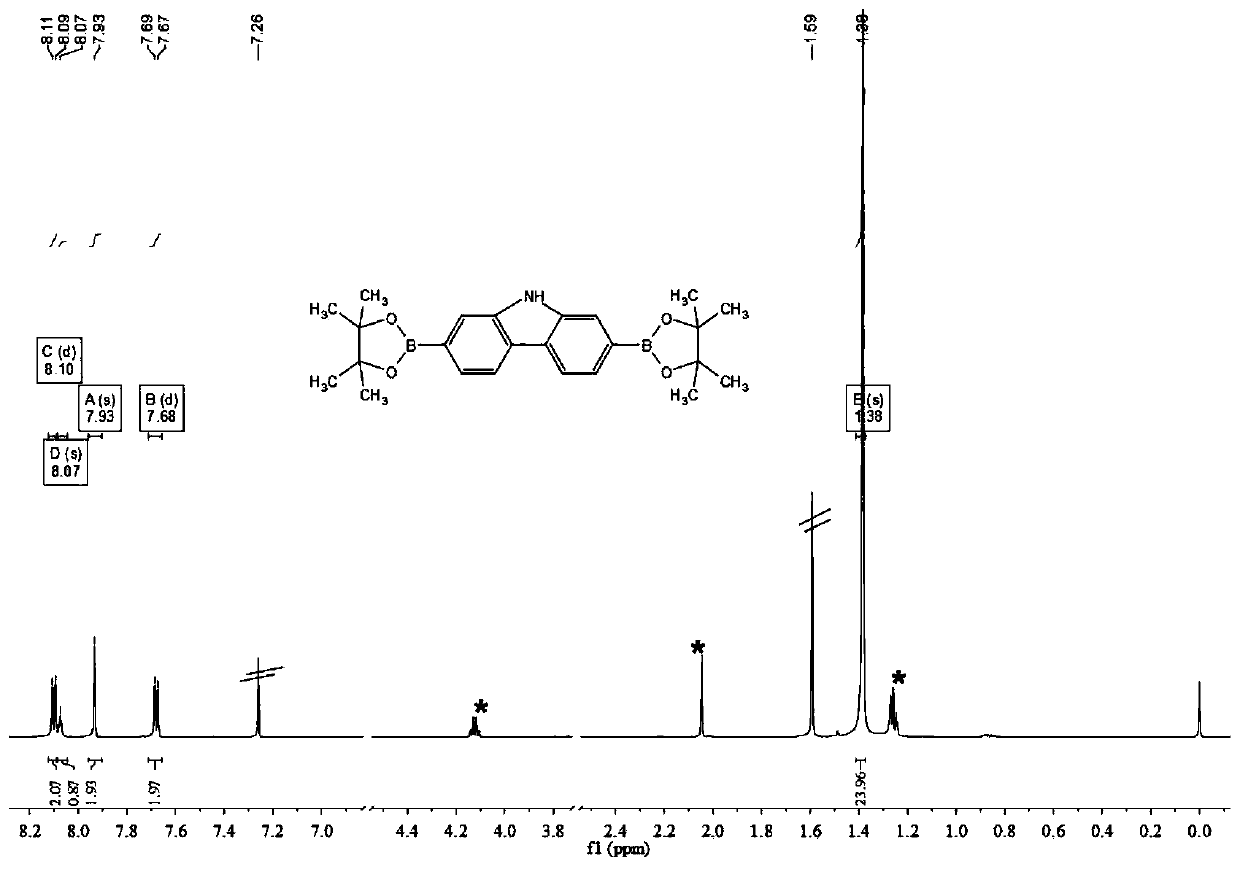
[0072] see Figure 1-10 and Figure 21 . Synthesis of intermediate 1-2
[0073]
[0074] Add compound Ⅰ-1 (2.0g, 6.2mmol), bis-pinacol boroester (3.6g, 14.3mmol), potassium acetate (3.1g, 31.0mmol) and 1,1' - Bisdiphenylphosphinoferrocenepalladium dichloride (226.0 mg, 0.3 mmol). Vacuum and replace with argon protection. Add 60 mL of 1,4-dioxane with a syringe, and place it in an oil bath at 95°C for 24 hours to react. Stop the reaction, spin off the solvent under reduced pressure, dissolve the residue in 120 mL of ethyl acetate, wash the organic phase with water, wash with saturated brine, dry over anhydrous sodium sulfate, and filter. The organic phase was spin-off, and the residue was separated by silica gel column chromatography using petroleum e...
Embodiment 2
[0085] This embodiment is basically the same as Embodiment 1, especially in that:
[0086] In this embodiment, a macrocyclic extended porphyrin compound containing a carbazole unit has a chemical structure of the general formula 2,
[0087]
[0088] . Its synthetic route is as follows:
[0089]
[0090] Synthesis of intermediate 2-2
[0091]
[0092] Add compound Ⅰ-1 (5.8g, 15.2mmol), bis-pinacol boroester (8.9g, 35.0mmol), potassium acetate (7.5g, 76.5mmol) and 1,1' - Bisdiphenylphosphinoferrocenepalladium dichloride (556.0 mg, 0.7 mmol). Vacuum and replace with argon protection. Add 120 mL of 1,4-dioxane with a syringe, and place it in an oil bath at 95°C for 24 hours to react. Stop the reaction, spin off the solvent under reduced pressure, dissolve the residue in 300 mL ethyl acetate, wash the organic phase with water, wash with saturated brine, dry over anhydrous sodium sulfate, and filter. The organic phase was spin-off, and the residue was separated by sil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com