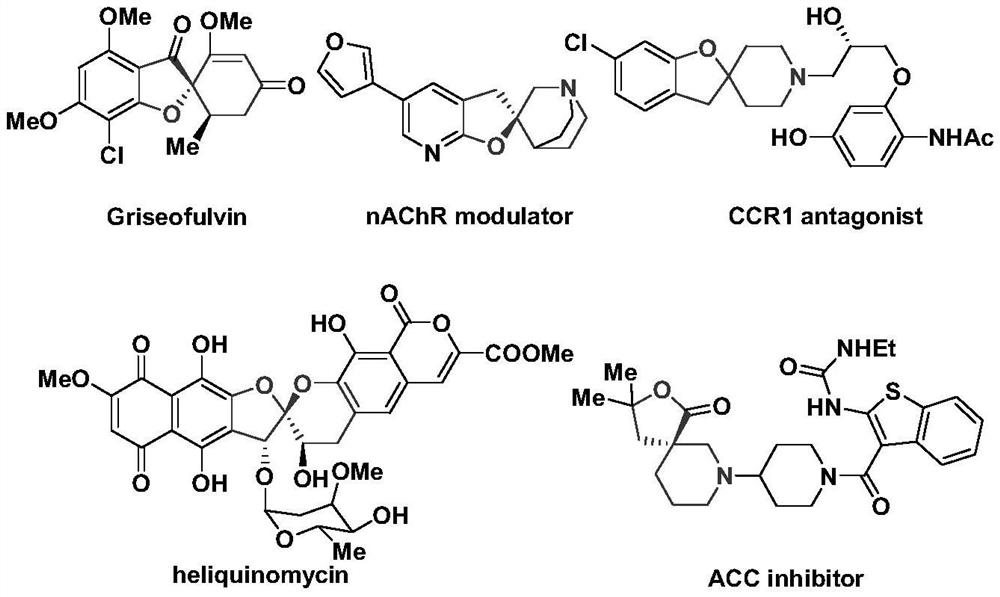
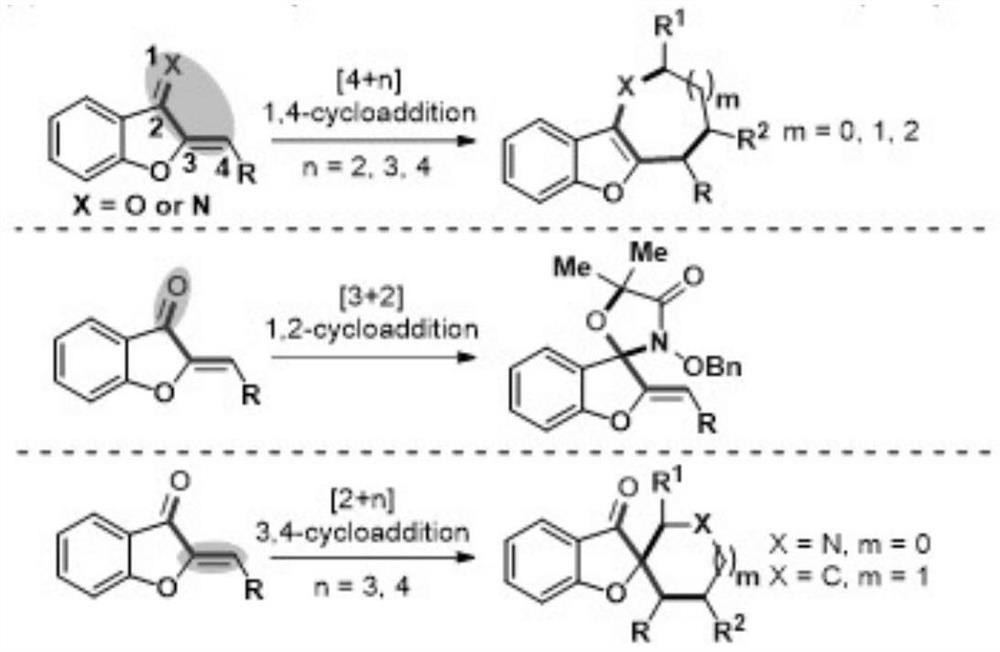
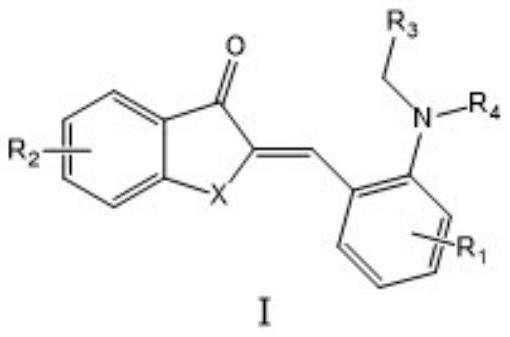
The method of anti-micheal addition reaction of arurone skeleton compound as acceptor
An addition reaction and compound technology, applied in the field of anti-Micheal addition reaction, to achieve the effects of wide substrate applicability, efficient and convenient reaction, and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]
[0042] Orange ketone compound 1a (0.1mmol) was subjected to trans-Michael addition in solvent (1.0mL) under the action of catalyst (20mol%, 0.02mmol) to obtain addition product 2a, and the conditions such as its catalyst, solvent and temperature were as follows: Table 1 shows:
[0043] Table 1
[0044] serial number catalyst solvent temperature(°C) time (h) Yield(%) 1 Cu(OTf) 2
DCE 120 1.5 23 2 Zn(OTf) 2
DCE 120 1.5 42 3 Mg(OTf) 2
DCE 120 1.5 n.r. 4 Sc(OTf) 3
DCE 120 1.5 94 5 TsOH·H 2 o
DCE 120 1.5 39 6 (-)-CSA DCE 120 1.5 72 7 TfOH DCE 120 1.5 57 8 Sc(OTf) 3
EtOH 120 1.5 84 9 Sc(OTf) 3
DCM 120 1.5 94 10 Sc(OTf) 3
toluene 120 1.5 75 11 Sc(OTf) 3
THF 120 1.5 43 12 Sc(OTf) 3
CH 3 CN
120 1.5 60 13 Sc(OTf) 3
DCM 80 3.5 89 14 Sc(OTf) 3
DCM 60 5.0 81 ...
Embodiment 2
[0054] Product chemical formula: C 20 h 20 NO 2
[0055] Molecular weight: 306.15
[0056] Structural formula:
[0057]
[0058] Yield: 43%
[0059] 1 H NMR (500MHz, CDCl 3 )δ7.70(dd, J=7.9,1.5Hz,1H),7.61–7.58(m,1H),7.13–7.07(m,2H),6.94(d,J=7.5Hz,1H),6.49(dd ,J=7.5,1.6Hz,1H),6.42(d,J=1.7Hz,1H),3.83(dd,J=10.0,5.6Hz,1H),3.55(td,J=8.7,2.1Hz,1H) ,3.35–3.23(m,2H),2.79(d,J=16.3Hz,1H),2.33(s,3H),2.05–1.97(m,1H),1.97–1.89(m,1H),1.77–1.69 (m,1H),1.42–1.32(m,1H);
[0060] 13 C NMR (126MHz, CDCl 3 )δ202.30, 172.09, 143.16, 138.08, 137.73, 129.04, 124.21, 121.97, 121.30, 116.92, 114.56, 113.65, 111.65, 84.32, 61.49, 47.58, 35.72, 25.54, 21.
Embodiment 3
[0062] Product chemical formula: C 20 h 20 NO 3
[0063] Molecular weight: 322.14
[0064] Structural formula:
[0065]
[0066] Yield: 71%
[0067] 1 H NMR (500MHz, CDCl 3 )δ7.70(d, J=7.8Hz, 1H), 7.59(t, J=7.8Hz, 1H), 7.09(dt, J=7.4, 3.1Hz, 2H), 6.95(d, J=8.2Hz, 1H), 6.24(dd, J=8.2, 2.4Hz, 1H), 6.16(d, J=2.5Hz, 1H), 3.80(s, 4H), 3.52(t, J=8.7Hz, 1H), 3.27( dt,J=16.6,5.1Hz,2H),2.77(d,J=16.2Hz,1H),2.06–1.97(m,1H),1.97–1.87(m,1H),1.76–1.66(m,1H) ,1.42–1.31(m,1H); 13 CNMR (126MHz, CDCl 3 )δ202.16,172.07,159.85,144.25,138.10,129.70,124.22,122.01,121.27,113.64,110.28,100.88,97.34,84.38,61.44,55.20,47.56,35.42,235.5
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com