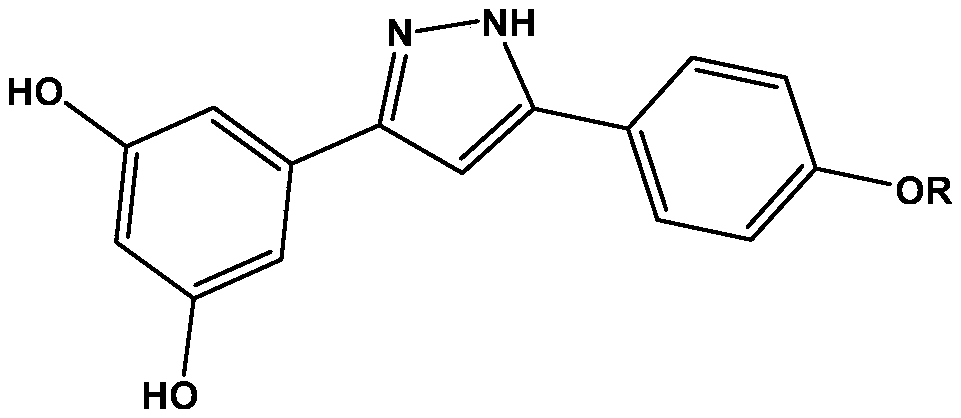
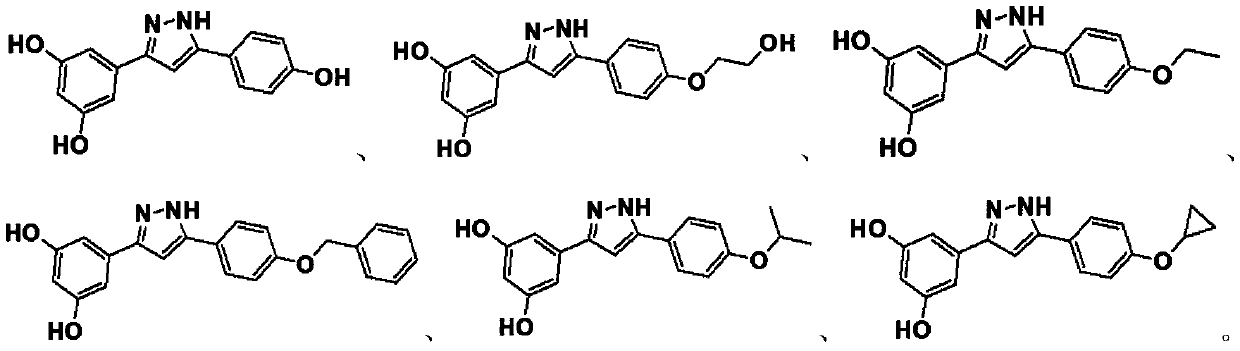
Arylpyrazole compound and application thereof
A technology of arylpyrazoles and compounds, applied in the field of chemical medicine, can solve problems such as vomiting and nausea, and achieve a good effect of inhibiting activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: the preparation of compound a
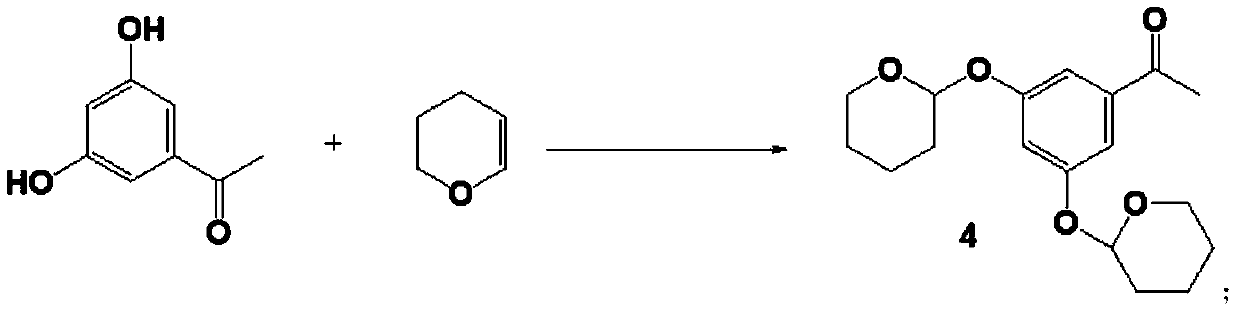
[0048] (1) Preparation of Intermediate 4
[0049]
[0050] In a 100 mL round bottom flask, add 3′,5′-dihydroxyacetophenone (1.52 g, 10 mmol), 3,4-dihydro-2H-pyran (1.68 g, 20 mmol), pyridine p-toluenesulfonic acid ( PPTs, 0.13g, 0.5mmol), mixed, added dichloromethane (50mL), dissolved, magnetically stirred, stopped after 24h at 40°C. The reaction solution was taken, layered with 10% sodium hydroxide (50 mL) and dichloromethane (50 mL), and the lower organic layer was taken. The extracted liquid was washed twice with 30 mL of saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain compound 4 (3.12 g, yield 97.5%). 1 H NMR (500MHz, CDCl 3 )δ7.26(s, 2H), 6.93(s, 1H), 5.58(s, 2H), 3.81(q, 3H), 3.62(t, 2H), 2.55(s, 3H), 1.98-1.90(m ,4H), 1.60-1.52(m,4H), 1.52-1.48(m,4H).
[0051] (2) Preparation of Intermediate 7
[0052]
[0053] In a 100mL round bottom fl...
Embodiment 2
[0060] Embodiment 2: the preparation of compound b
[0061] (1) The preparation of intermediate 4 is the same as in Example 1
[0062] (2) Preparation of Intermediate 6
[0063]
[0064] In a 100mL round bottom flask, add p-hydroxybenzaldehyde (1.22g, 10mmol), 2-bromoethanol (1.25g, 10mmol), K 2 CO 3 (0.69g, 5mmol), mix, add DMF (50mL), dissolve, dissolve, magnetically stir, stop after 24h at 40°C. The reaction solution was taken, layered with 10% sodium hydroxide (50 mL) and dichloromethane (50 mL), and the lower organic layer was taken. The extracted liquid was washed twice with 30 mL of saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain compound 6 (1.35 g, yield 81.3%). 1 H NMR (500MHz, CDCl 3 )δ9.86(s, 1H), 7.72(d, 2H, J=8.3Hz), 7.17(d, 2H, J=8.2Hz), 3.93(m, 3H), 3.82(m, 3H), 3.21( m, 1H).
[0065] (3) Preparation of intermediate 8 and final product b with reference to Example 1, the total yield o...
Embodiment 3
[0068] Embodiment 3: the preparation of compound c
[0069] The synthesis was carried out with reference to the preparation method of compound b in Example 2, bromoethane was used instead of 2-bromoethanol in the preparation of intermediate 6, and the total yield of compound c was 60.9%. The structural formula is as follows:
[0070]
[0071] ESI MS:297.7[M+H] +1 , 1 H NMR (500MHz, CDCl 3 )δ9.97(s,1H),9.52(s,1H),9.23(s,2H),7.66(d,2H,J=8.0Hz),6.83(m,3H),6.61(m,2H), 6.13 (s, 1H), 3.82 (m, 2H), 1.31 (m, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com