Atrial septostomy device and atrial septostomy system
A technology of atrial septum and stoma, applied in the field of medical devices, can solve problems such as loss of shunt function, tissue shedding, device shedding, etc., and achieve the effects of avoiding device falling off, increasing the heating range, and reducing incomplete ablation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
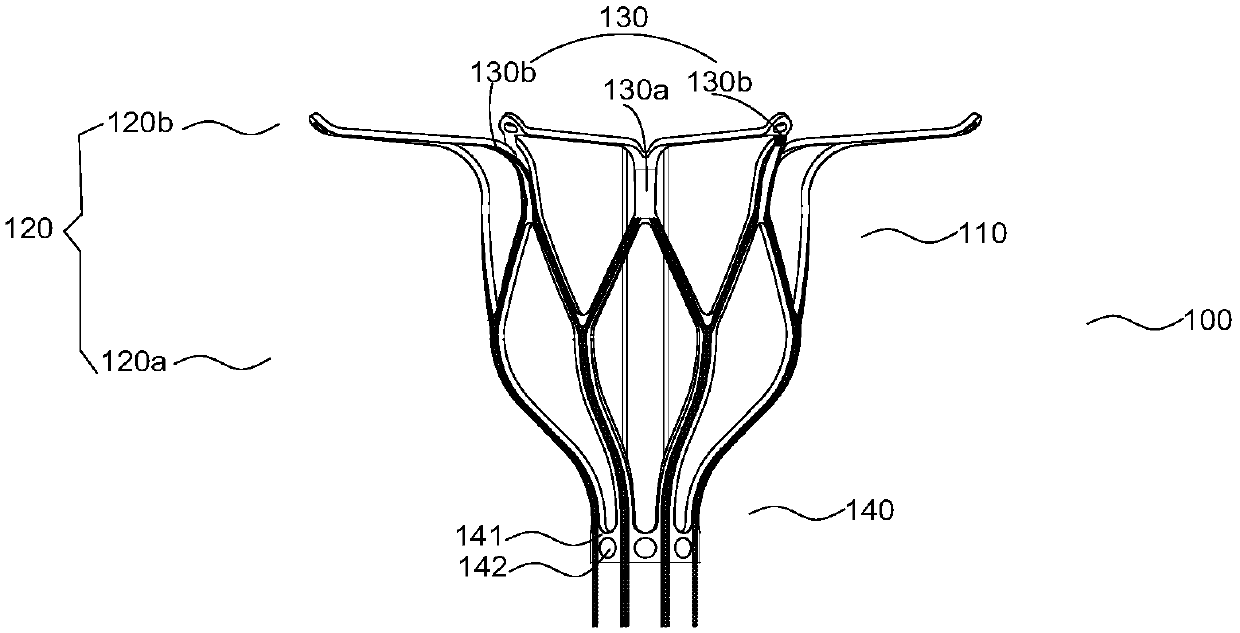
[0076] Example 1, such as Figure 1-3 As shown, an atrial septostomy device 100 includes a support part 110 that passes through the atrial septum and radially expands the atrial septal tissue to form a perforation, an extension part 120 connecting the support part 110, and an ablation part 130; The portion 120 includes a compensating extension 120a for extending the length of the support portion 110 and / or a positioning extension 120b for positioning the support portion 110 . The ablation part 130 includes at least two sets of ablation elements 130a, 130b for ablation of atrial septal tissue, which are electrically connected to the ablation power supply on the support part 110 or / and the extension part. Wherein, the compensation extension part 120a is arranged in the axial direction of the support part 110, and the positioning extension part 120b extends outward from the support part 110 and presses against the interatrial septal tissue; the ablation element 130a arranged on t...
Embodiment 2
[0105] Example 2, such as Figure 4As shown, this embodiment is improved on the basis of Embodiment 1.
[0106] An atrial septostomy device 100 , comprising a support part 110 that passes through the atrial septum and expands radially to spread the atrial septal tissue to form a perforation, and an extension part 120 connected to the support part 110 .
[0107] The difference between this embodiment and Embodiment 1 is: the extension part 120 in this embodiment includes two positioning extension parts 120b for positioning the support part 110; A positioning extension part 120b is provided respectively, and a set of ablation elements 130a and 130b for ablation of interatrial septal tissue are provided on the support part 110 and the extension part 120 respectively.
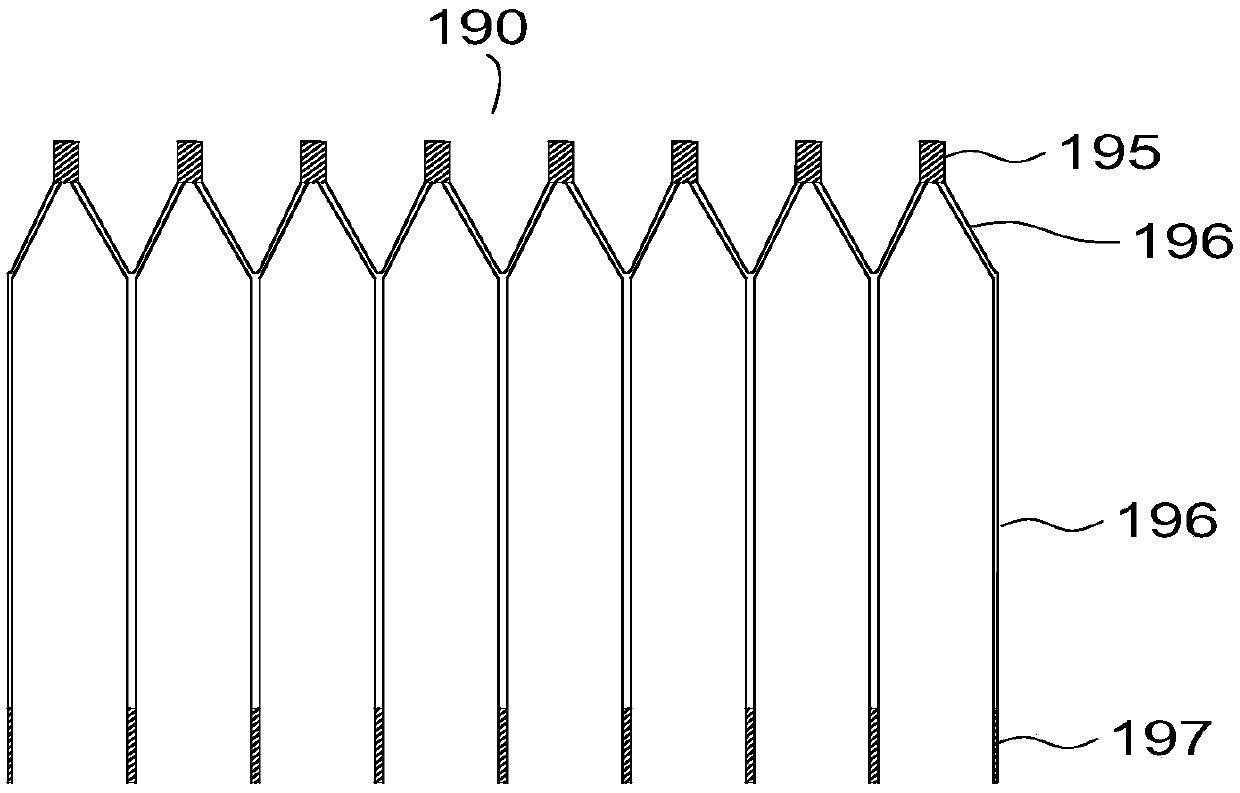
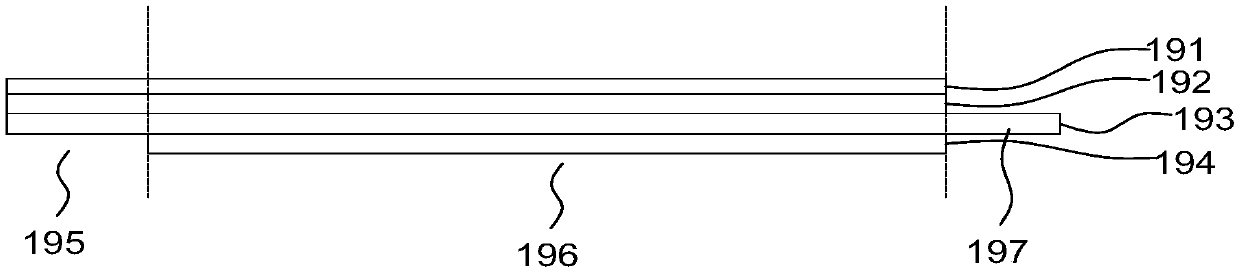
[0108] Such as Figure 4 As shown, in this embodiment, both the support part 110 and the positioning extension part 120b are mesh structures formed by weaving metal wires, and the support part 110 and the positio...
Embodiment 3
[0122] Embodiment 3, this embodiment is improved on the basis of Embodiment 2.
[0123] Such as Figure 5-7 As shown, an atrial septostomy device 100 includes a support part 110 that passes through the atrial septum and radially expands to spread the atrial septal tissue to form a perforation, and an extension part 120 that connects the support part 110 . The most important difference from the structure of embodiment 2 is that no thrombus arresting mechanism is provided.
[0124] Such as Figure 5 As shown, another different structure is: the supporting part 110 is a drum-shaped structure formed by a concave surface of revolution of the generatrix, and the extending part 120 includes two positioning extending parts 120b for positioning the supporting part 110 . The two positioning extensions 120b are respectively located at the distal end and the proximal end of the support portion 110 . Both the support part 110 and the positioning extension part 120b in this embodiment ar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com