Physiological poison metabolic kinetic model of adults orally exposed to DEHP
A kinetic model, an adult technique for use in public health to address uncertainty, extrapolation of prediction results, difficult organ or tissue isolation and extraction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
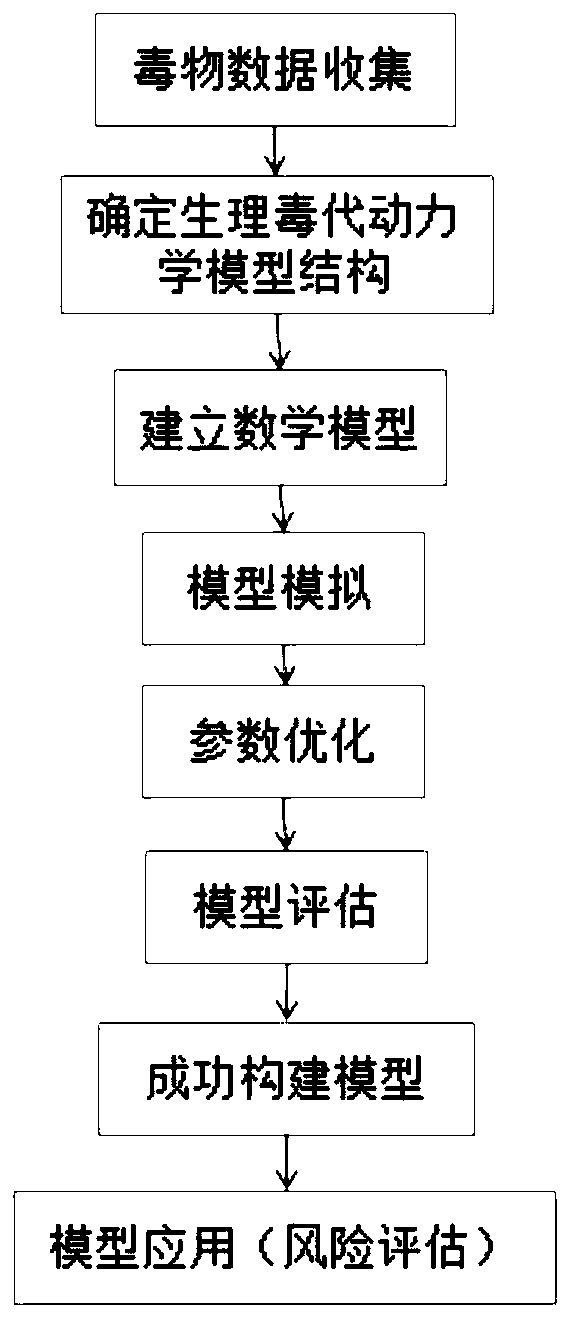
[0049] This embodiment discloses a method for constructing a physiological toxicokinetic model of adult oral exposure to DEHP, including:
[0050] S1: Determine the kinetics of absorption, distribution, metabolism and excretion of orally ingested DEHP in humans;
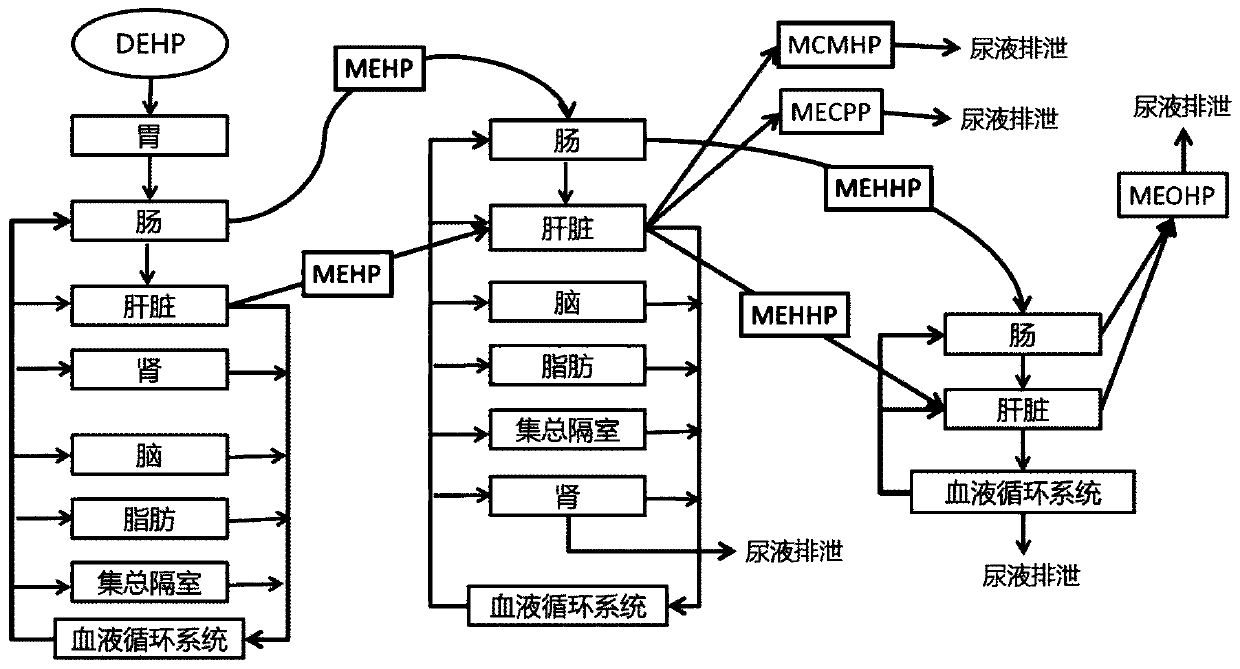
[0051] S2: Determine the structure of a physiological toxicokinetic model for oral exposure of adults to DEHP;
[0052] S3: Establish mathematical models and write differential equations;
[0053] S4: Acquire human physiological parameters, DEHP biochemical parameters and DEHP toxicokinetic parameters;
[0054] S5: DEHP physiological toxicokinetic model simulation and parameter optimization;
[0055] S6: After the parameters of the DEHP physiological toxicokinetic model are optimized, the obtained DEHP physiological toxicokinetic model is evaluated.
[0056] For the construction process of the specific DEHP physiological toxicokinetic model, see figure 1 ,Proceed as follows:
[0057] 1. Determination of the kine...
Embodiment 2
[0078] This example discloses a physiological toxicokinetic model of adults exposed to DEHP, which is obtained according to the construction method described in Example 1. The application of this model is as follows:
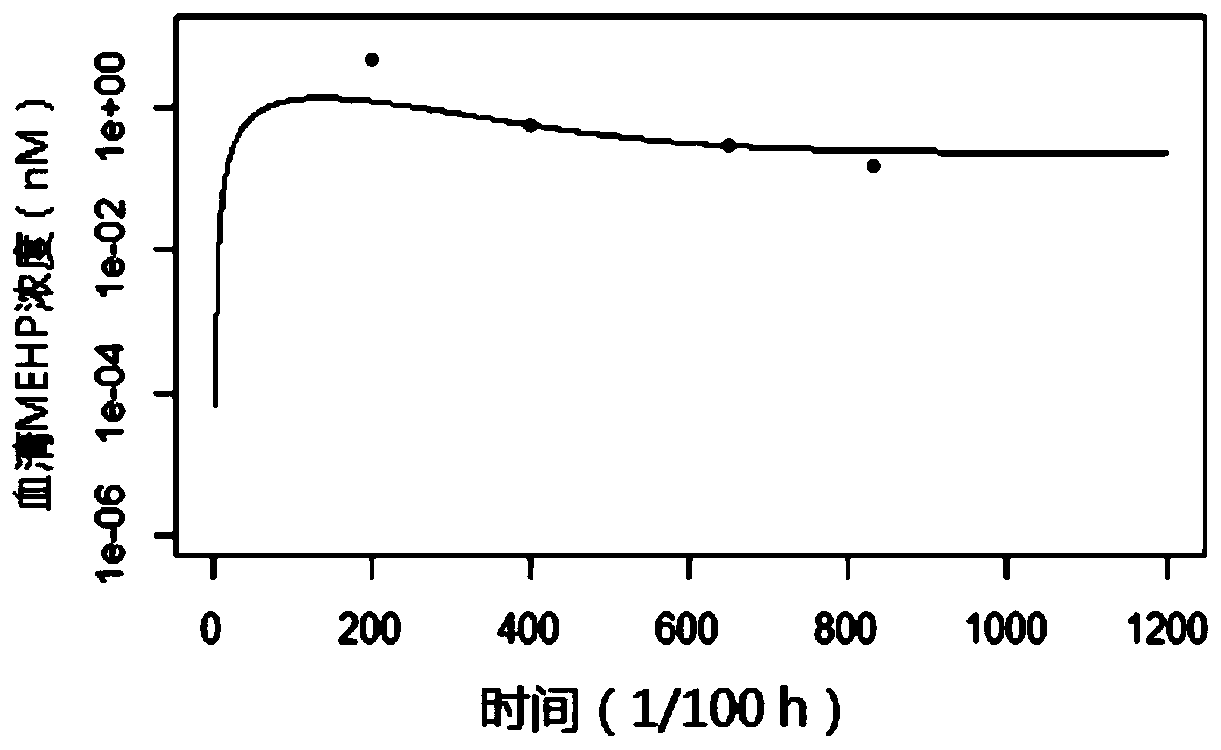
[0079] Simulate the long-term oral intake of DEHP for adults: In order to simulate the actual meal situation of adults, the daily oral intake dose is divided into 3 portions, which are taken at 0, 6, and 12 hours respectively, and the duration of each intake is about 6 minutes, and no intake for 12-24 hours after that, set the model running time to 6*24 hours. The intake used in the model application is 4.44 μg / kg bw / day, and the MEHP concentration-time curve in the serum is predicted. The results are as follows Figure 7 Shown; predict the concentration-time curve of MEHHP in urine as Figure 8 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com