Preparation method of high-purity micafungin intermediate
A technology of micafungin and body formula, which is applied in the preparation of 4-[5-isoxazol-3-yl]benzoic acid and the field of preparation of high-purity micafungin intermediates, can solve the problem of low product purity and reagent Expensive, many waste solvents and other problems, to achieve the effect of high product purity and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
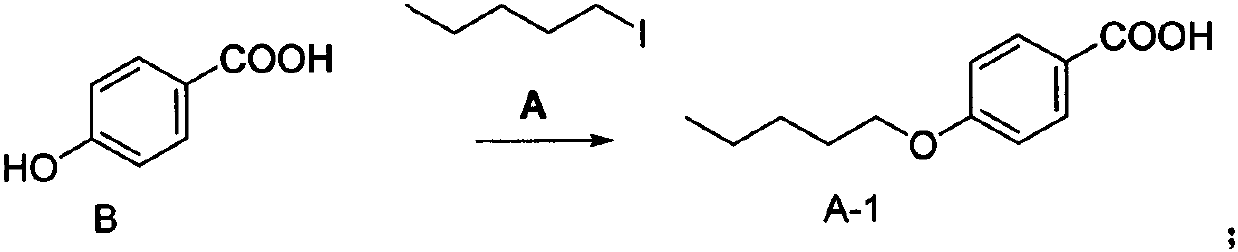
[0024] Example 1 Preparation of Compound A-1
[0025] Add 5 g of p-hydroxyacetophenone (compound of formula B), 7.9 g of iodopentane (compound of formula A), and 6 g of anhydrous potassium carbonate into 50 mL of DMF, stir at 60°C for 4 hours. Detect the reaction. After the reaction is completed, cool to room temperature, add petroleum ether (50 mL) and water (50 mL) in sequence, and keep the petroleum ether layer. The petroleum ether layer was washed successively with 0.5 mol / L sodium hydroxide solution and saturated brine, dried by adding anhydrous magnesium sulfate, filtered and concentrated to obtain 7 g of compound A-1 with a yield of 93% and a purity of 95% by HPLC.
Embodiment 2
[0026] Example 2 Preparation of Compound A-3
[0027] Compound A-1 (4g), p-aldehyde benzoic acid (4.1g, compound of formula A-2), and 1.3g of sodium hydroxide were sequentially added to water (20ml) and ethanol (8ml), and stirred at 40°C for 4 hours. After the reaction was completed, the reaction solution was cooled in an ice bath, 10% dilute hydrochloric acid was added to adjust the pH to 2, and the solid was collected by filtration. The solid was stirred in 45 ml of ethanol at room temperature, and compound A-3 (5.5 g) was obtained after filtration, with a yield of 85% and a purity of 95.7% by HPLC.
Embodiment 3
[0028] Example 3 Preparation of Compound A-4
[0029] Compound A-3 (2.7 g), TsNHOH (N-p-toluenesulfonyl hydroxylamine, 11 g), sodium hydroxide (2.5 g) were dissolved in 50 ml of a mixed solution of methanol and water (volume ratio 4:1). Stir at 43°C for 35 hours. After the reaction is completed, add 10% dilute hydrochloric acid to the reaction solution under ice water cooling to adjust the pH to 2, and filter. Add 80ml of ethanol to the obtained solid, stir and beat at room temperature, wash the filter cake, and filter to obtain compound A-4, further add the compound to 60ml of ethanol, beat at room temperature, filter and dry to obtain compound A-4 (2.2g), yield 80%. The HPLC content is 98.5%, the maximum single hetero HPLC content is <0.8%, and the isomer impurity is <0.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com