Method for targeting Hsp27 to inhibit enterovirus type A71 infection and related applications
A virus infection, EV-A71 technology, applied in the direction of antiviral agents, biochemical equipment and methods, pharmaceutical formulations, etc., can solve the problem of limited, promoting or limiting EV-A71 host factors have not been fully determined, host-virus interaction The underlying mechanism is complex and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1, EV-A71 infection and 2-DE analysis of uninfected RD cells
[0069] RD cells, which have been widely used to study the mechanism of enterovirus infection, were used for the proteomic analysis in this example. Prior art studies on the kinetics of EV-A71 infection have shown that viral RNA synthesis and protein translation are highly active in phage 6 to 9 hours post-infection (p.i.) when cells are infected at a multiplicity of infection (MOI) of 1 or 10; In addition, the progeny virions were gradually packaged and released during the same period (Lu, J., He, Y.Q., Yi, L.N., Zan, H., Kung, H.F. and He, M.L. (2011) Viral kinetics of enterovirus 71 in human abdominal osarcoma cells . World journal of gastroenterology, 17, 4135-4142). In the present invention, in order to identify host factors that respond to early viral replication, proteins were extracted and p.i. applied to proteomic analysis after 6 hours. At this point, EV-A71 infection is established with h...
Embodiment 2
[0075] Embodiment 2, western blot analysis protein
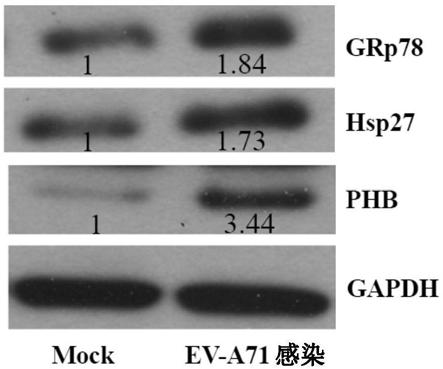
[0076] Western blot analysis was performed using whole protein extracts of RD cells that were mock or EV-A71-infected at an MOI of 10 at 6 hours post-infection. Three altered chaperones (GRp78, PHB and Hsp27) associated with cellular stress responses were selected for validation. Consistent with the observations in the 2-DE analysis, all three proteins were found to be upregulated ( Figure 1C , Figure 1D ).
Embodiment 3
[0077] Example 3, Hsp27 expression up-regulation in cells infected with EV-A71
[0078] In order to reveal the dynamic changes of Hsp27 during EV-A71 infection, this example analyzed the mRNA and protein levels of Hsp27 during virus infection. Cellular mRNA and protein were extracted from EV-A71 infected cells at different time points. Such as Figure 2A As shown, the mRNA level of Hsp27 was significantly up-regulated at p.i. 6 hours and 9 hours, and the up-regulation was about 100% at 6 hours. Likewise, when the viral protein VP1 was expressed at relatively high levels, Hsp27 protein levels also increased by 40% to 50% at 6, 9 and 12 hours p.i. ( Figure 2B ).
[0079] These results were further confirmed in Hela cells ( Figure 3A ), indicating that EV-A71 infection induces Hsp27 expression.
[0080] Furthermore, new synthesis of viral proteins was first detected at 6 hours p.i. A significant increase in Hsp27 was also found at the same time point ( Figure 2B ).
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com