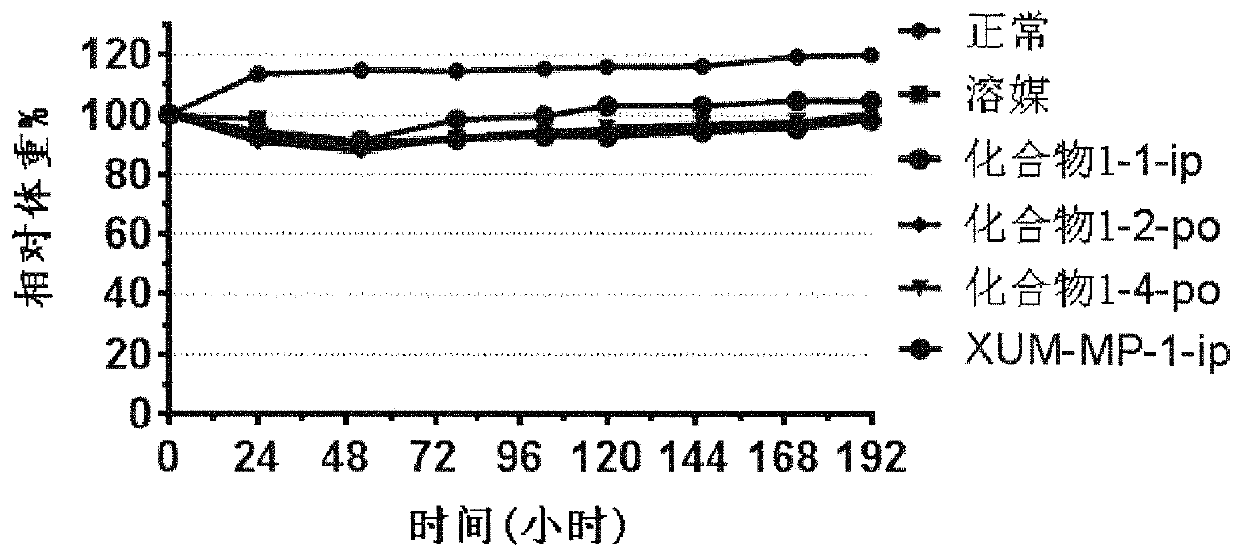
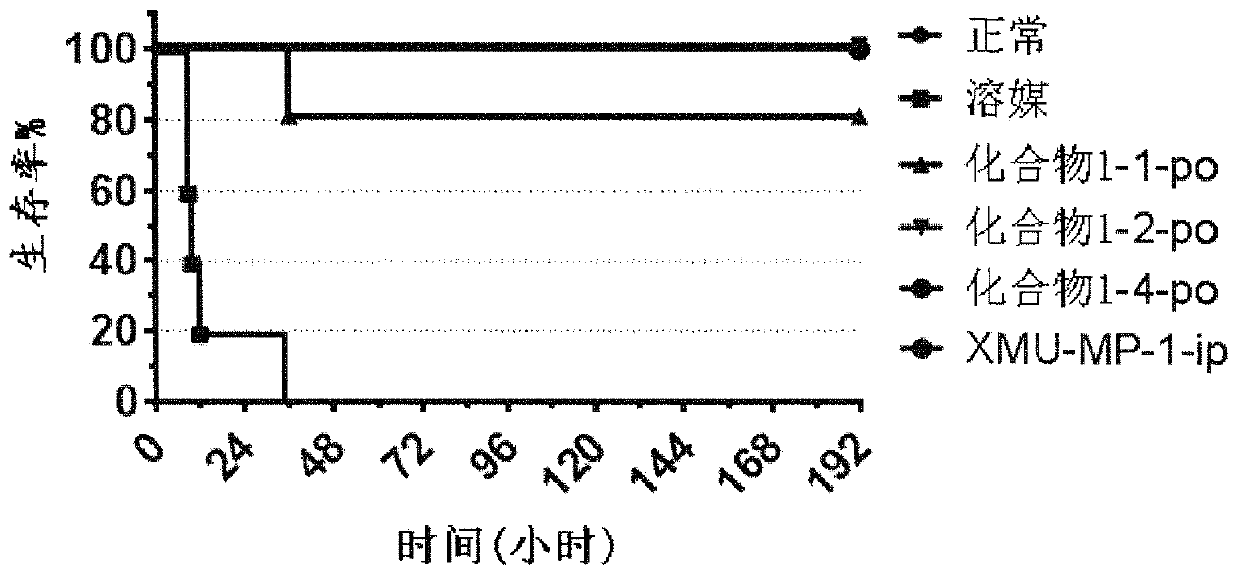
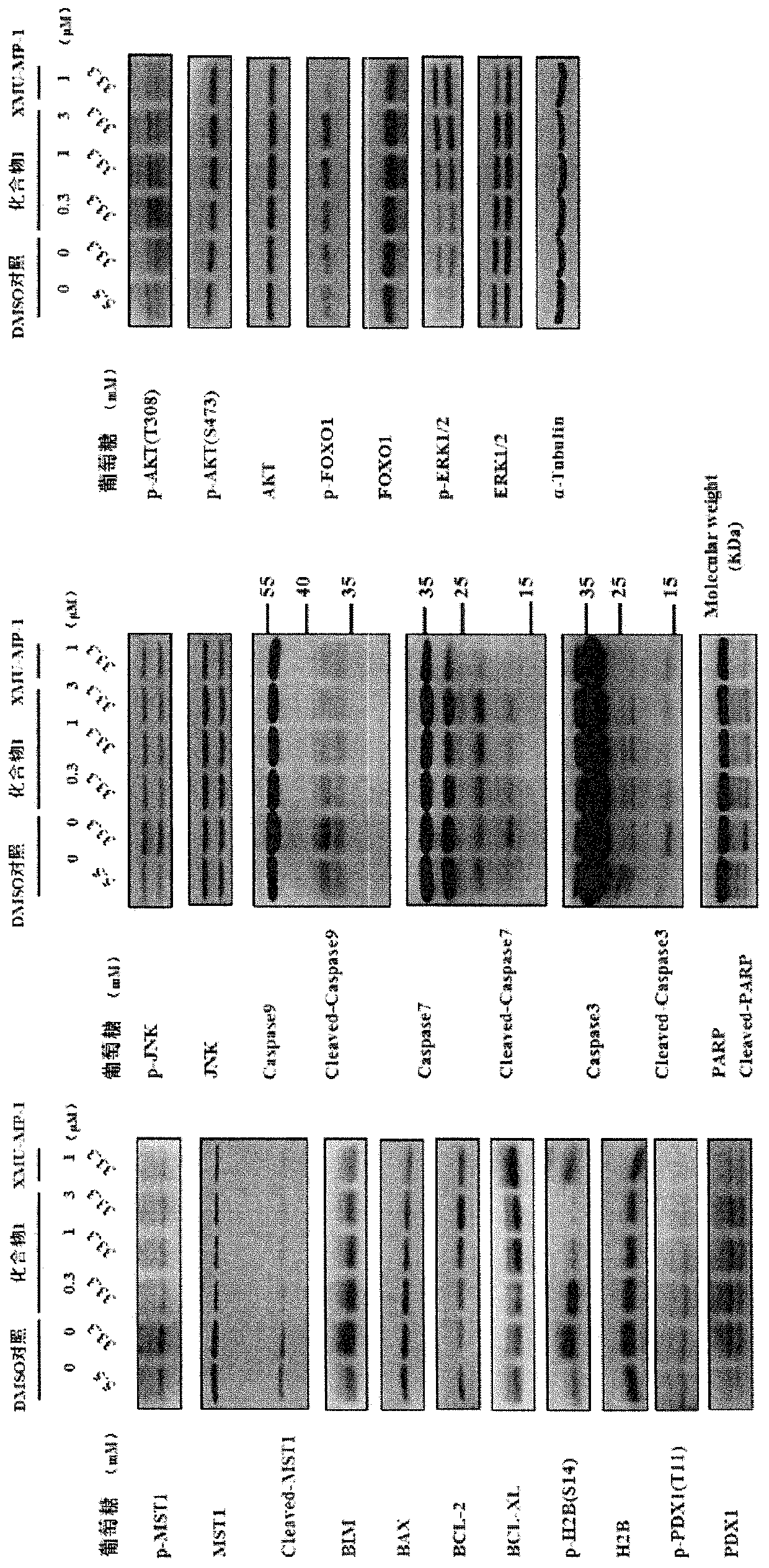
MST1 kinase inhibitor and application thereof
A technology of kinase inhibitors and solvates, which is applied in the field of MST1 kinase inhibitors and their uses, and can solve the problems of not showing selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0129] Example 1: 4-((7-(2,6-difluorophenyl)-5,8-dimethyl-6-oxo-5,6,7,8-tetrahydropteridin-2-yl ) Amino)benzenesulfonamide
[0130]
[0131] 2-Amino-2-(2,6-difluorophenyl)acetic acid methyl ester (A2): Add 3-2-amino-2-(2,6-difluorophenyl)acetic acid (2.0 g) was added methanol (30 mL), followed by the dropwise addition of thionyl chloride (1.2 mL) under ice-cooling. The reaction system was reacted overnight at 85°C. After the reaction, the system was evaporated to dryness under reduced pressure to obtain a white solid, which was directly used in the next step.
[0132] Methyl 2-((2-chloro-5-nitropyrimidin-4-yl)amino)-2-(2,6-difluorophenyl)acetate (A3): Add 2-amino- Add acetone (30 ml) and potassium carbonate (2.2 g) after 2-(2,6-difluorophenyl) methyl acetate (2 g), then cool the system to -10°C with an ice-salt bath, then slowly add 2,4-Dichloro-5-nitropyrimidine (3.1 g) in acetone. The reaction was stirred overnight at room temperature. After the reaction was com...
Embodiment 2
[0136] Example 2: 4-((5,8-dimethyl-6-oxo-7-phenyl-5,6,7,8-tetrahydropteridin-2-yl)amino)benzenesulfonate Amide
[0137]
[0138] The synthesis of the compound of Example 2 was accomplished using a procedure similar to that described in Example 1. MS (ESI) m / z (M+1)+: 425.14.
Embodiment 3
[0139] Example 3: 4-((7-benzyl-5,8-dimethyl-6-oxo-5,6,7,8-tetrahydropteridin-2-yl)amino)benzenesulfonate Amide
[0140]
[0141] Synthesis of the compound of Example 3 was accomplished using a procedure similar to that described in Example 1. MS (ESI) m / z (M+1)+: 439.15.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com