Combination of drugs for use in the treatment of Alzheimer's disease
一种阿尔茨海默氏、药学的技术,应用在5-HT6受体拮抗剂及其药学上可接受的盐领域,能够解决没有任何治愈和治疗痴呆等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0017] Embodiments of the present invention are disclosed below. The first embodiment is denoted El, the second E2, and so on.
[0018] E1 a 5-HT 6 Receptor antagonists or pharmaceutically acceptable salts thereof for the treatment of Alzheimer's disease by improving or increasing the effect of acetylcholinesterase inhibitor therapy wherein Alzheimer's disease patients carry one or two ApoE4 etc. bit gene.
[0019] E2 In one embodiment of E1, 5-HT 6 The receptor antagonist is selected from the group consisting of Idalupyridine, AVN-211 and RVT-101, or the 5-HT 6 A pharmaceutically acceptable salt of a receptor antagonist.
[0020] E3 In one embodiment of E1 or E2, 5-HT 6 The receptor antagonist is Idalupridine or a pharmaceutically acceptable salt thereof.
[0021] E4 In one embodiment of E1 or E3, 5-HT 6 The receptor antagonist is Idalupridine hydrochloride.
[0022] E5 In one embodiment of E1 or E2, 5-HT 6 The receptor antagonist is AVN-211 or a pharmaceutically acc...
example 1
[0100] Example 1: Binding Affinity Determination and Results for Idalupridine
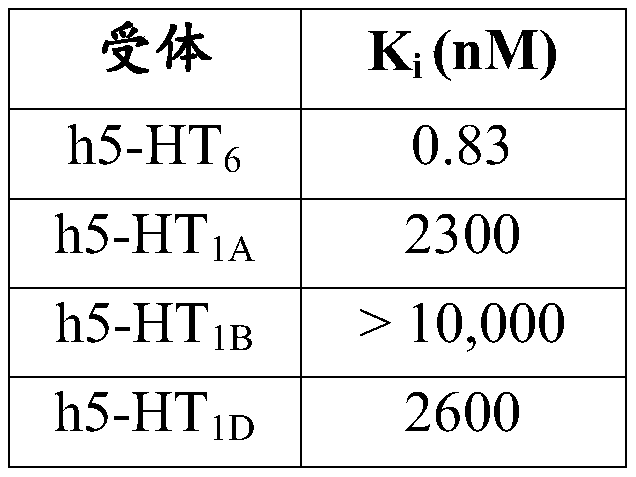
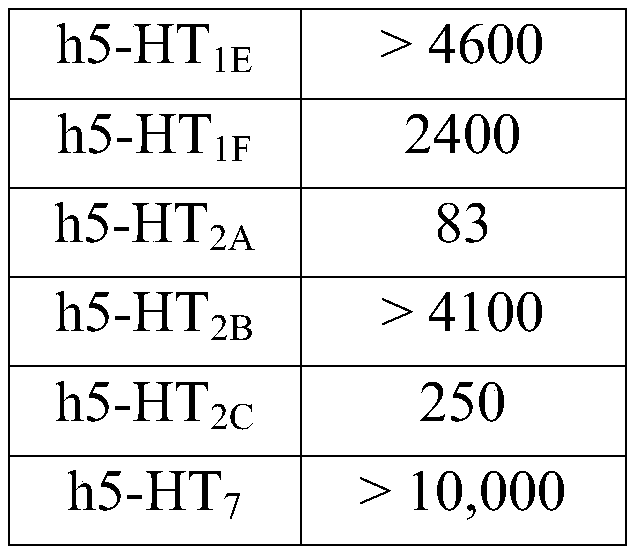
[0101] Previously, as Arnt J, et al. Lu AE58054, a 5-HT 6 receptor antagonist, reverse cognitive impairment induced by subchronic phencyclidine in a novel object recognition test in rats. [a 5-HT 6 Receptor antagonist Lu AE58054 reverses subchronic phencyclidine-induced cognitive impairment in a novel object recognition test in rats] Int J Neuropsychopharmacol 2010; 13:1021-1033 Determination of the 5-HT binding affinity of idalupridine. Reported results showing that N-(2-(6-fluoro-1H-indol-3-yl)-ethyl)-3-(2,2,3,3-tetrafluoropropoxy)-benzylamine is effective and selective human 5-HT 6 Receptor Antagonist, Its Effect on Human 5-HT 6 The receptor, as well as other human 5-HT receptor subtypes, has the following affinities:
[0102]
[0103]
[0104] Table 1: Idalupridine Inhibition of 5-HT Receptors
example 2
[0105] Example 2: Phase III Study
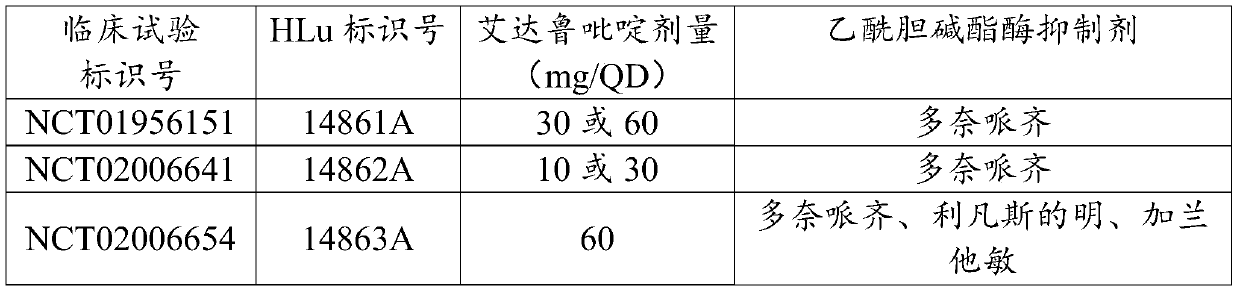
[0106] The Phase III program was initiated by H.Lundbeck A / S (HLu) and consisted of three 24-week trials in patients over 50 years of age with mild-moderate AD (MMSE 12-22 at screening) , double-blind, parallel-group, placebo-controlled, fixed-dose (10, 30, and 60 mg QD) idalupridine as adjuvant AChEI study consisted of:
[0107]
[0108] Table 2: Phase III studies
[0109] Change from baseline to week 24 in the primary endpoint on the Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-cog). This endpoint addresses the primary objective of the study, which was to establish the efficacy of idalupridine as adjunctive therapy to donepezil in the symptomatic treatment of patients with mild-moderate AD.
[0110] Key secondary endpoints included change from baseline to week 24 in the Alzheimer's Disease Collaborative Study-Detailed Record of Activities of Daily Living (ADCS-ADL) total score.
[0111] The Mini-Mental State Examinat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com