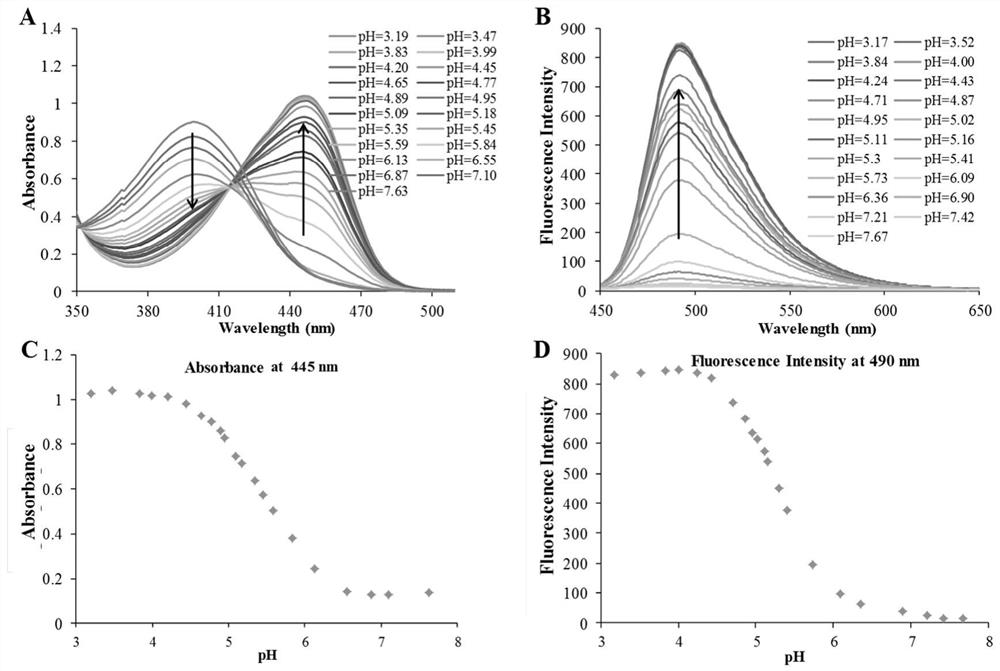
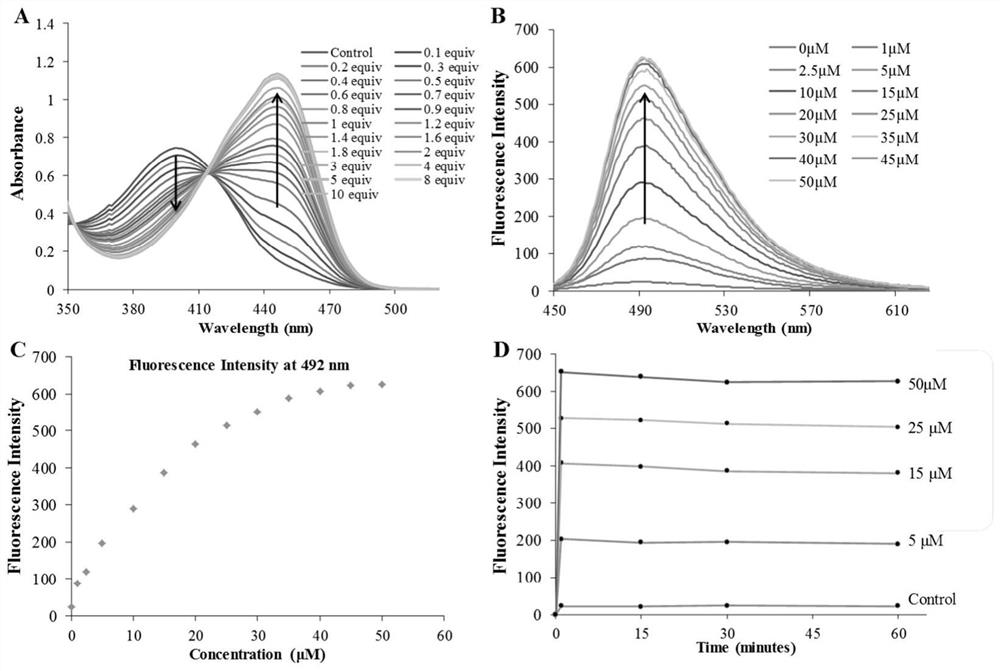
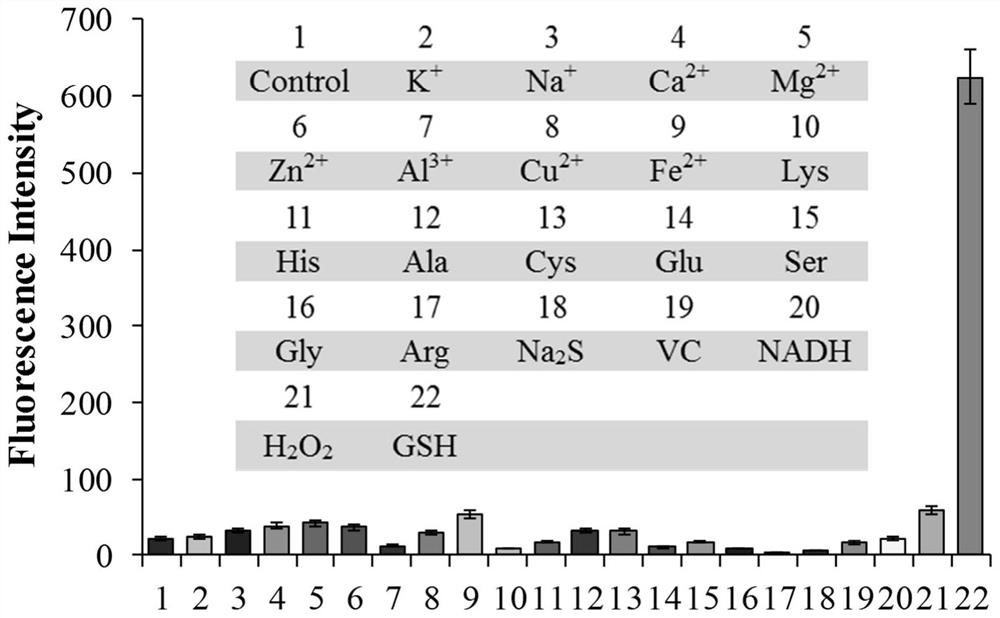
β-carboline-cycloenone derivatives based on dual response of ph and gsh and uses thereof
A technology of cycloalkenone and derivatives, applied in the preparation of anti-tumor diagnosis and treatment agents, β-carboline-cycloalkenone derivatives and the field of preparation, can solve the problems of instability in the body, easy metabolism, slow color development and the like , to achieve the effect of enhancing anti-tumor proliferation and metastatic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1 (6-oxocyclohex-1-en-1-yl)methyl (1-(3,4,5-trimethoxyphenyl)-9H-pyrido[3,4-b]ind Indol-3-yl) carbamate (I 1 ) or bis(6-oxocyclohex-1-en-1-yl)methyl(1-(3,4,5-trimethoxyphenyl)-9H-pyrido[3,4-b]ind Indol-3-yl) carbamate (II 1 ) preparation
[0075]
[0076] (1) Preparation of 1-(3,4,5-trimethoxyphenyl)-9H-pyrido[3,4-b]indole-3-carbonylhydrazide (2a)
[0077] Compound 1-(3,4,5-trimethoxyphenyl)-9H-pyrido[3,4-b]indole-3-carboxylic acid methyl ester (1a) (19.6g, 50mmol) was dissolved in 80mL After methanol, 176.4 mL of 85% hydrazine hydrate (75 g, 1.50 mol) was added and refluxed for 4-5 h. The reaction was monitored by TLC. After the reaction liquid was cooled to 0° C., it was vacuum pumped to obtain a light brown solid. A large amount of cold water was added to the filtrate, and the solid continued to precipitate. After suction filtration and vacuum drying, 16.5 g of a light brown solid was obtained, with a yield of 84.2%.
[0078] (2) Preparation of 1-(3,4,5...
Embodiment 2
[0086] Example 2 (6-oxocyclohex-1-en-1-yl)methyl (1-(p-tolyl)-9H-pyrido[3,4-b]indol-3-yl)aminomethyl Ester (Ⅰ 2 ) or bis(6-oxocyclohex-1-en-1-yl)methyl(1-(p-tolyl)-9H-pyrido[3,4-b]indol-3-yl)aminomethyl Ester (II 2 ) preparation
[0087] Preparation of 1-(p-tolyl)-9H-pyrido[3,4-b]indole-3-carbonylhydrazide (2b)
[0088] Referring to the synthesis method of (2a) in Example 1, (1a) in the method was replaced by (1b), and finally a light yellow solid (2b) was obtained with a yield of 80.9%.
[0089] Preparation of 1-(p-tolyl)-9H-pyrido[3,4-b]indole-3-yl azide (3b)
[0090] Referring to the synthesis method of (3a) in Example 1, (2a) in the method was replaced by (2b), and finally a light yellow solid (3b) was obtained with a yield of 87.5%.
[0091] Preparation of 1-(p-tolyl)-9H-pyrido[3,4-b]indol-3-amine (4b)
[0092] Referring to the synthesis method of (4a) in Example 1, (3a) in the method was replaced by (3b), and finally a pale yellow solid (4b) was obtained with a yie...
Embodiment 3
[0095]Example 3 (6-oxocyclohex-1-en-1-yl)methyl (1-(3-methoxyphenyl)-9H-pyrido[3,4-b]indole-3- base) carbamate (Ⅰ 3 ) or bis(6-oxocyclohex-1-en-1-yl)methyl(1-(3-methoxyphenyl)-9H-pyrido[3,4-b]indole-3- base) carbamate (II 3 ) preparation
[0096] Preparation of 1-(3-methoxyphenyl)-9H-pyrido[3,4-b]indole-3-carbonylhydrazide (2c)
[0097] Referring to the synthesis method of (2a) in Example 1, (1a) in the method was replaced by (1c), and finally a light yellow solid (2c) was obtained with a yield of 81.3%.
[0098] Preparation of 1-(3-methoxyphenyl)-9H-pyrido[3,4-b]indole-3-ylazide (3c)
[0099] Referring to the synthesis method of (3a) in Example 1, (2a) in the method was replaced by (2c), and finally a light yellow solid (3c) was obtained with a yield of 88.4%.
[0100] Preparation of 1-(3-methoxyphenyl)-9H-pyrido[3,4-b]indol-3-amine (4c)
[0101] Referring to the synthesis method of (4a) in Example 1, (3a) in the method was replaced by (3c), and finally a pale yellow so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com