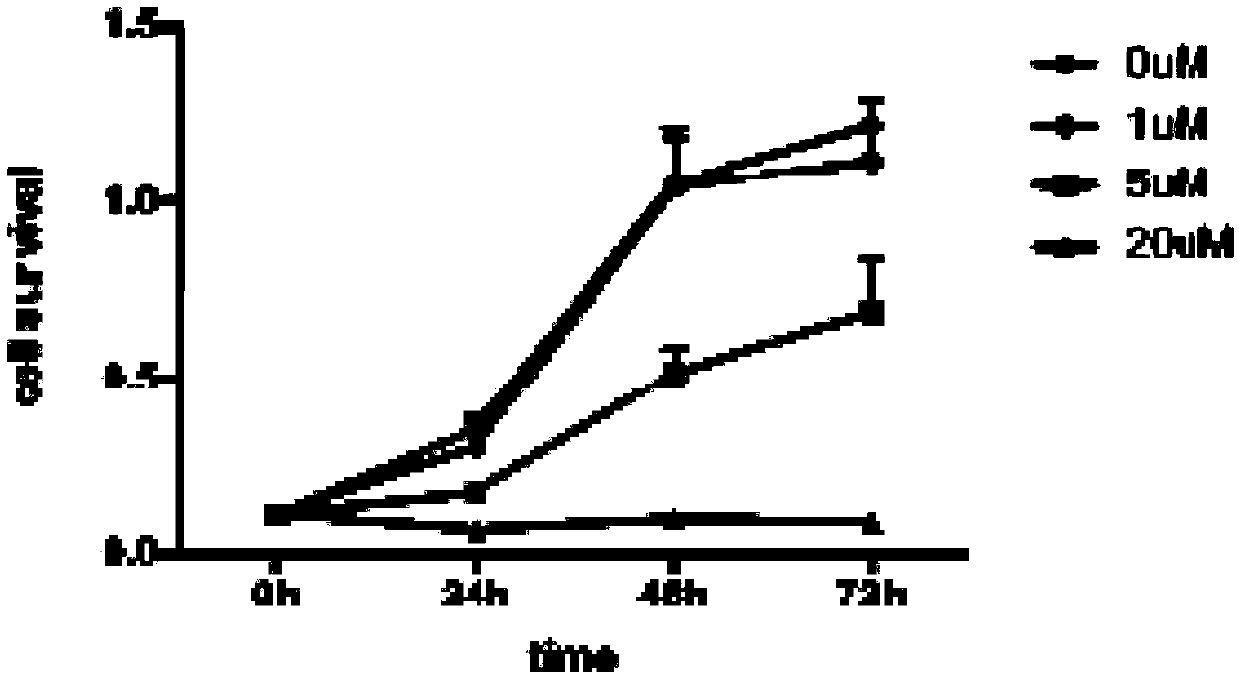
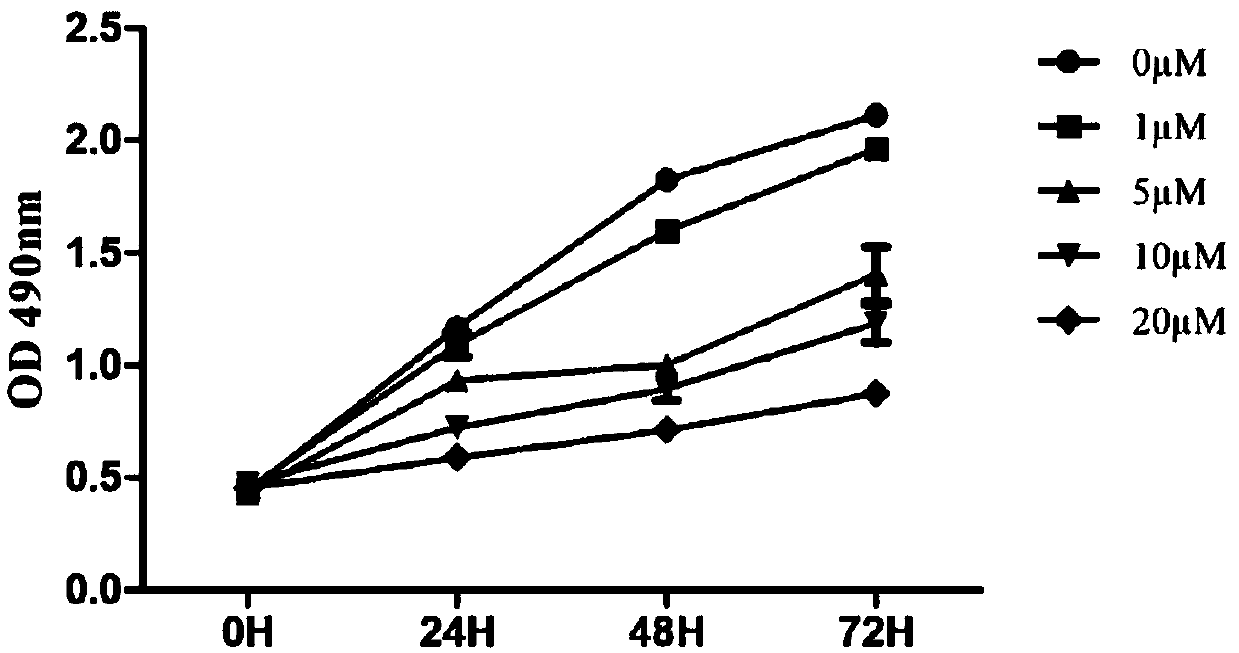
Anti-tumor compound aiming at Fyn-CD147 signal pathway target point, and preparation method and application of anti-tumor compound
A signaling pathway and anti-tumor drug technology, which is applied in the preparation of carbon-based compounds, organic compounds, anti-tumor drugs, etc., can solve the problems of high prices and few anti-tumor drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The preparation method of the above-mentioned antitumor compound according to an embodiment of the present invention comprises the following steps: mixing 4-methoxy-3-hydroxyacetophenone, 4-biphenylformaldehyde and alkali solution, stirring at room temperature for 24 to 30 hours, Concentrate and dry to obtain the above antitumor compound.
[0032] In a specific example, the molar ratio of 4-methoxy-3-hydroxyacetophenone to 4-biphenylformaldehyde is (0.8-1.2):1.
[0033] In a specific example, the alkaline solution is an aqueous sodium hydroxide solution, wherein the ratio of the mass of sodium hydroxide to the volume of water is (16-18) mg:1 mL.
[0034] In a specific example, the temperature of the alkaline solution is 0° C. to 10° C., and using a low-temperature alkaline solution for preparation can prevent excessive exothermic heat during the reaction.
[0035] In a specific example, the following step is further included: recrystallizing the antitumor compound with...
Embodiment 1
[0046] Add sodium hydroxide aqueous solution (NaOH 290mg / H2 O 15mL) and water (2mL), cooled to below 10°C with an ice-water bath, added 4-methoxy-3-hydroxyacetophenone (997mg, 6mmol) and stirred for 30 minutes, then added 4-biphenylcarbaldehyde (1.09g , 6 mmol) was stirred for 1 hour. Then rise to room temperature and stir for 26 hours, concentrate the reaction solution, add 200 mL of ethyl acetate, wash with 3N hydrochloric acid and water successively, then dry with anhydrous magnesium sulfate, filter and concentrate and recrystallize with ethanol to obtain the target product 1.4 as a yellow solid. g, based on dividing the actual yield by the theoretical yield to obtain a yield of 71%. The NMR spectrum information is: 1 H NMR (400MHz, CDCl 3 )δ7.85(d, J=15.6Hz, 1H), 7.72(d, J=8.3Hz, 2H), 7.69–7.61(m, 6H), 7.57(d, J=15.6Hz, 1H), 7.47( t, J=7.5Hz, 2H), 7.38(t, J=7.3Hz, 1H), 6.95(d, J=9.0Hz, 1H), 3.99(s, 3H); 13 C NMR (125MHz, DMSO) δ187.9, 152.8, 147.1, 142.8, 142.3, 139.7,...
Embodiment 2
[0048] Add sodium hydroxide aqueous solution (NaOH 190mg / H 2 O 15mL) and water (2mL), cooled to below 10°C with an ice-water bath, added 4-methoxy-3-hydroxyacetophenone (997mg, 6mmol) and stirred for 30 minutes, then added 4-biphenylcarbaldehyde (1.09g , 6 mmol) was stirred for 1 hour. Then rise to room temperature and stir for 20 hours, concentrate the reaction solution, add 200 mL of ethyl acetate, wash with 3N hydrochloric acid and water successively, then dry with anhydrous magnesium sulfate, filter and concentrate and recrystallize with ethanol to obtain the target product 1.1 as a yellow solid g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com