A class of asymmetric V-shaped organic dye sensitizers, preparation method and applications thereof
An organic dye, asymmetric technology, applied in organic dyes, organic chemistry, chemical instruments and methods, etc., can solve the problems of restricting large-scale commercial production, complex synthesis of metal complexes, and high price, and achieve enhanced light harvesting efficiency , suitable energy gap and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
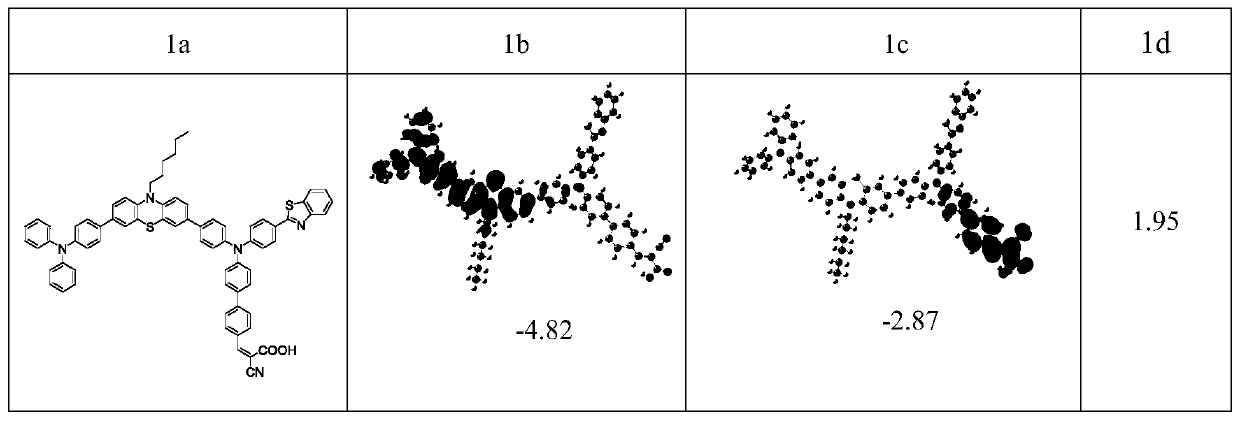
[0029] Asymmetric V-type Dye Molecule V1 Containing Triphenylamine π Bridge
[0030]
[0031] Under ice-water bath at 0°C, triphenylamine (4.9g, 20mmol) was dissolved in 50ml of anhydrous DMF solution, and 50mL of POCl was slowly added dropwise 3 . Under the protection of nitrogen, the mixture was slowly heated to 87° C. and then reacted for 1 h. After cooling to room temperature, the reaction mixture was poured into an ice bath and neutralized with NaOH, then extracted with chloroform. The combined organic layers were dried over anhydrous magnesium sulfate and filtered. In case of concentrating the organic layer, column chromatography on silica gel with ethyl acetate-petroleum ether mixture (1:10 / v) as eluent gave white purified compound 1a. Yield 90%. 1 H NMR (500MHz, Chloroform-d) δ9.88(s,0H),7.88–7.82(m,1H),7.36–7.30(m,1H),7.30–7.20(m,4H),7.18–7.11(m ,1H).
[0032]
[0033] Compound 1a (2.73g, 10mmol), 2-aminothiophenol (1.1mL, 10mmol) and NMP (20mL) were heated...
Embodiment 2
[0043] Asymmetric V-type Dye Molecule V2 Containing Phenylcarbazole π Bridge
[0044]
[0045] Under 0°C ice-water bath, 9-phenyl-9H-carbazole (2.43 g, 10 mmol) was dissolved in 25 ml of anhydrous DMF solution, and 25 mL of POCl3 was slowly added dropwise. Under the protection of nitrogen, the mixture was slowly heated to 85° C. and reacted for 2 h. After cooling to room temperature, the reaction mixture was poured into an ice bath and neutralized with NaOH, then extracted with chloroform, and dried over anhydrous sodium sulfate. Separation and purification by silica gel column chromatography finally obtained the target product 1b, 1.788 g of a yellow solid, and the reaction yield was 55%. 1 H NMR (500MHz, Chloroform-d) δ9.88(s,0H),8.00(dd,J=7.6,1.5Hz,1H),7.96–7.90(m,1H),7.58–7.52(m,1H), 7.47–7.42 (m, 1H), 7.29–7.19 (m, 2H).
[0046]
[0047] Compound 1b (1.37g, 5mmol), 2-aminothiophenol (0.55mL, 5mmol) and NMP (10mL) were heated in an oil bath at a temperature of 110°...
Embodiment 3
[0057] Asymmetric V-type Dye Molecule V2 Containing Phenylcarbazole π Bridge
[0058]
[0059] Under 0°C ice-water bath, 9-phenyl-9H-carbazole (2.43 g, 10 mmol) was dissolved in 25 ml of anhydrous DMF solution, and 25 mL of POCl3 was slowly added dropwise. Under the protection of nitrogen, the mixture was slowly heated to 90° C. and reacted for 1.5 h. After cooling to room temperature, the reaction mixture was poured into an ice bath and neutralized with NaOH, then extracted with chloroform, and dried over anhydrous sodium sulfate. Separation and purification by silica gel column chromatography finally obtained the target product 1b, 1.788 g of a yellow solid, and the reaction yield was 55%. 1 H NMR (500MHz, Chloroform-d) δ9.88(s,0H),8.00(dd,J=7.6,1.5Hz,1H),7.96–7.90(m,1H),7.58–7.52(m,1H), 7.47–7.42 (m, 1H), 7.29–7.19 (m, 2H).
[0060]
[0061] Compound 1b (1.37g, 5mmol), 2-aminothiophenol (0.55mL, 5mmol) and NMP (10mL) were heated in an oil bath at a temperature of 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com