Chimeric antigen receptors targeting FLT3
A chimeric antigen receptor and monoclonal antibody technology, which is applied in the field of chimeric antigen receptor targeting FLT3, can solve the problem of short response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0376] Example 1: Determination of Kinetics and Affinity of Human FLT3 / FLT3 Antibody Interaction at 37°C
[0377] This example measures the kinetics and affinity of various anti-FLT3 antibodies at 37°C. All experiments were performed on a Biacore T200 surface plasmon resonance biosensor (GE Lifesciences, Piscataway NJ).
[0378] Sensor chip preparation was performed at 25°C with a running buffer of 10 mM HEPES, 150 mM NaCl, 0.05% (v / v) Tween-20, pH 7.4. Anti-human Fc sensor chips were obtained by activating all flow cells of the Biacore CM4 sensor chip with a 1:1 (v / v) mixture of 400 mM EDC and 100 mM NHS at a flow rate of 10 μL / min for 7 minutes. Dilute anti-human Fc reagent (goat anti-human IgG Fc, SouthernBiotech cat#2081-01) to 30 μg / mL in 10 mM sodium acetate, pH 4.5, and inject into all flow cells at 20 μL / min for 7 minutes. All flow cells were blocked with 100 mM ethylenediamine in 150 mM borate buffer, pH 8.5, at 10 μL / min for 7 minutes.
[0379] Experiments were...
example 2
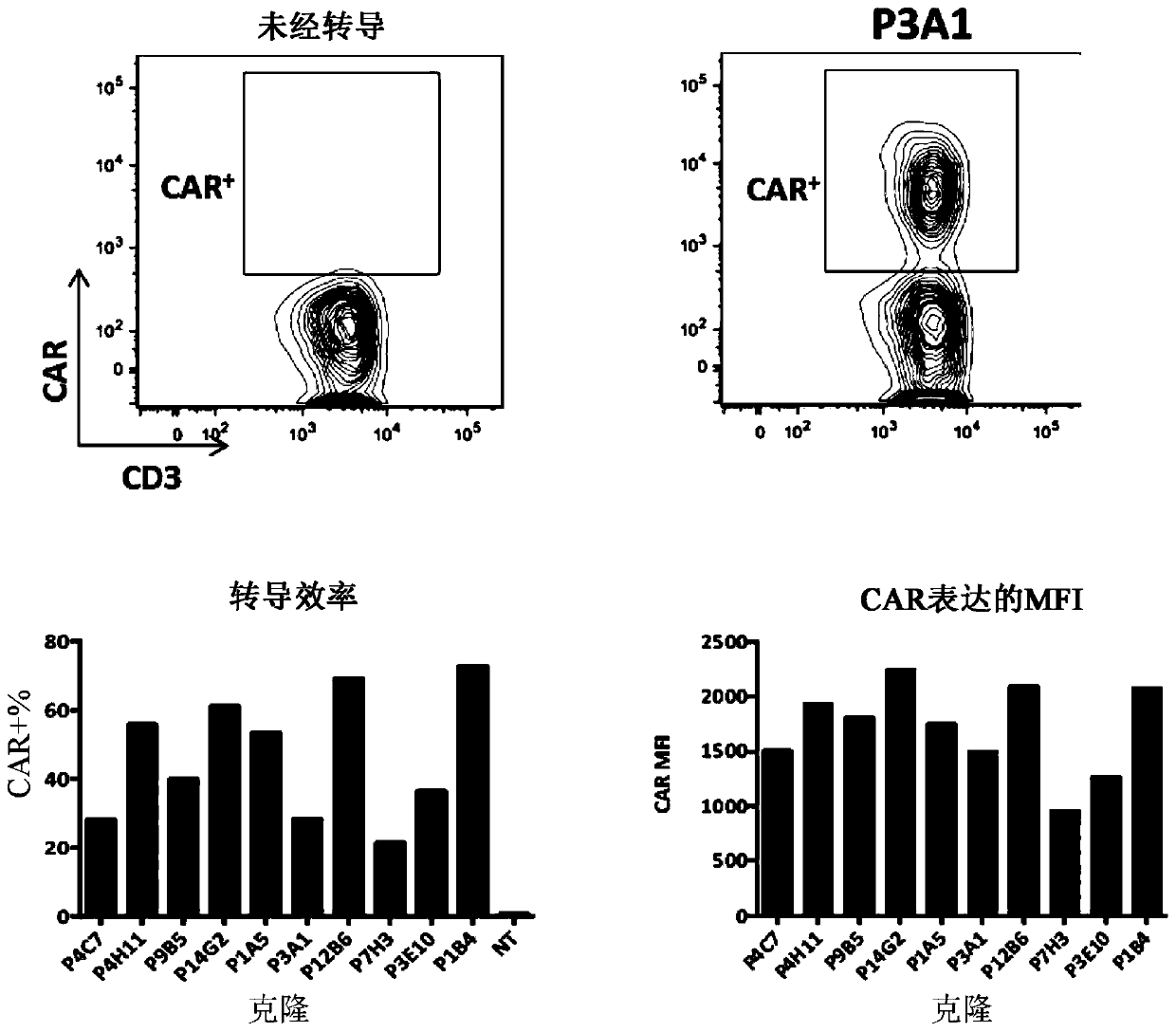
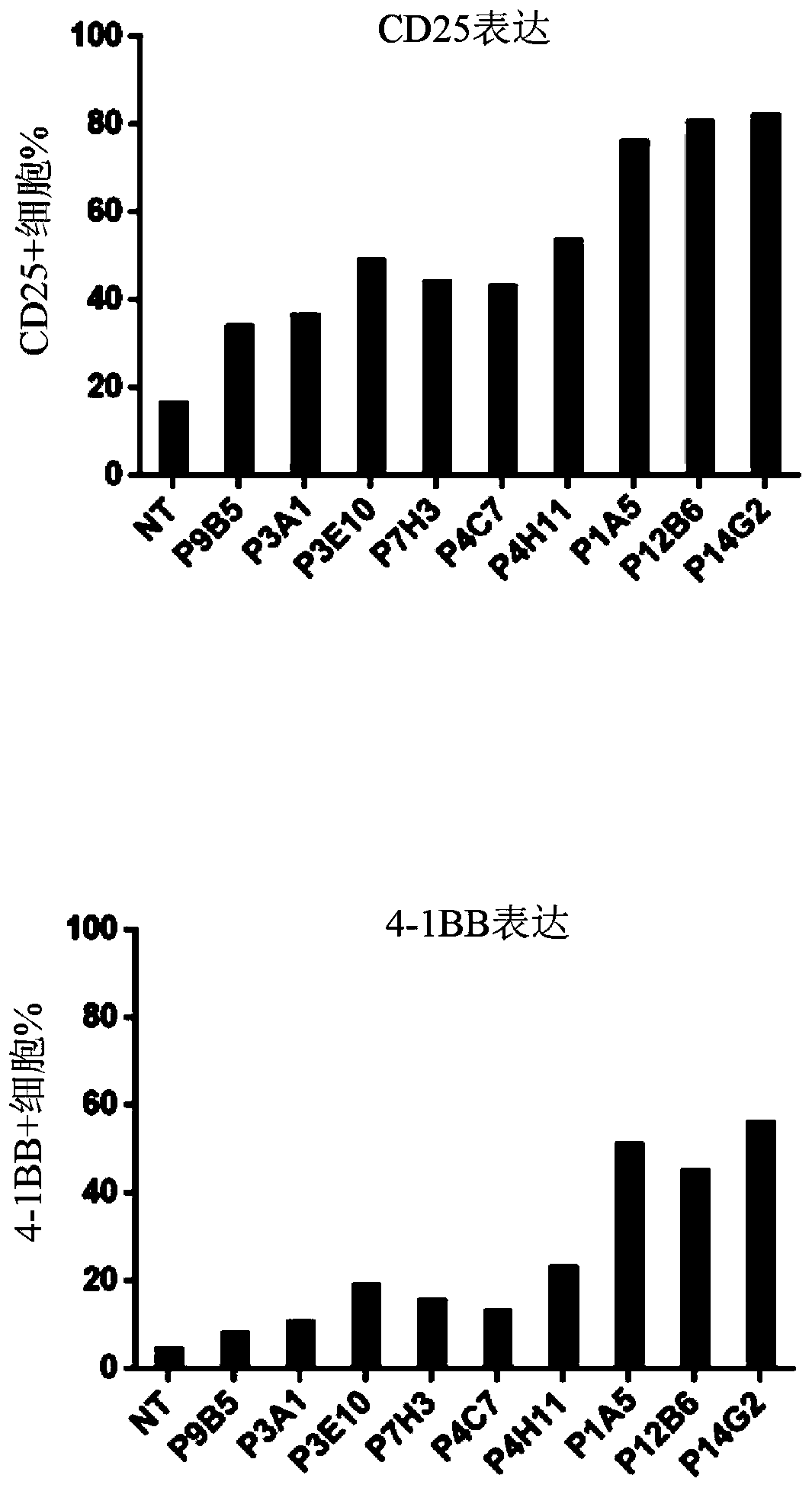
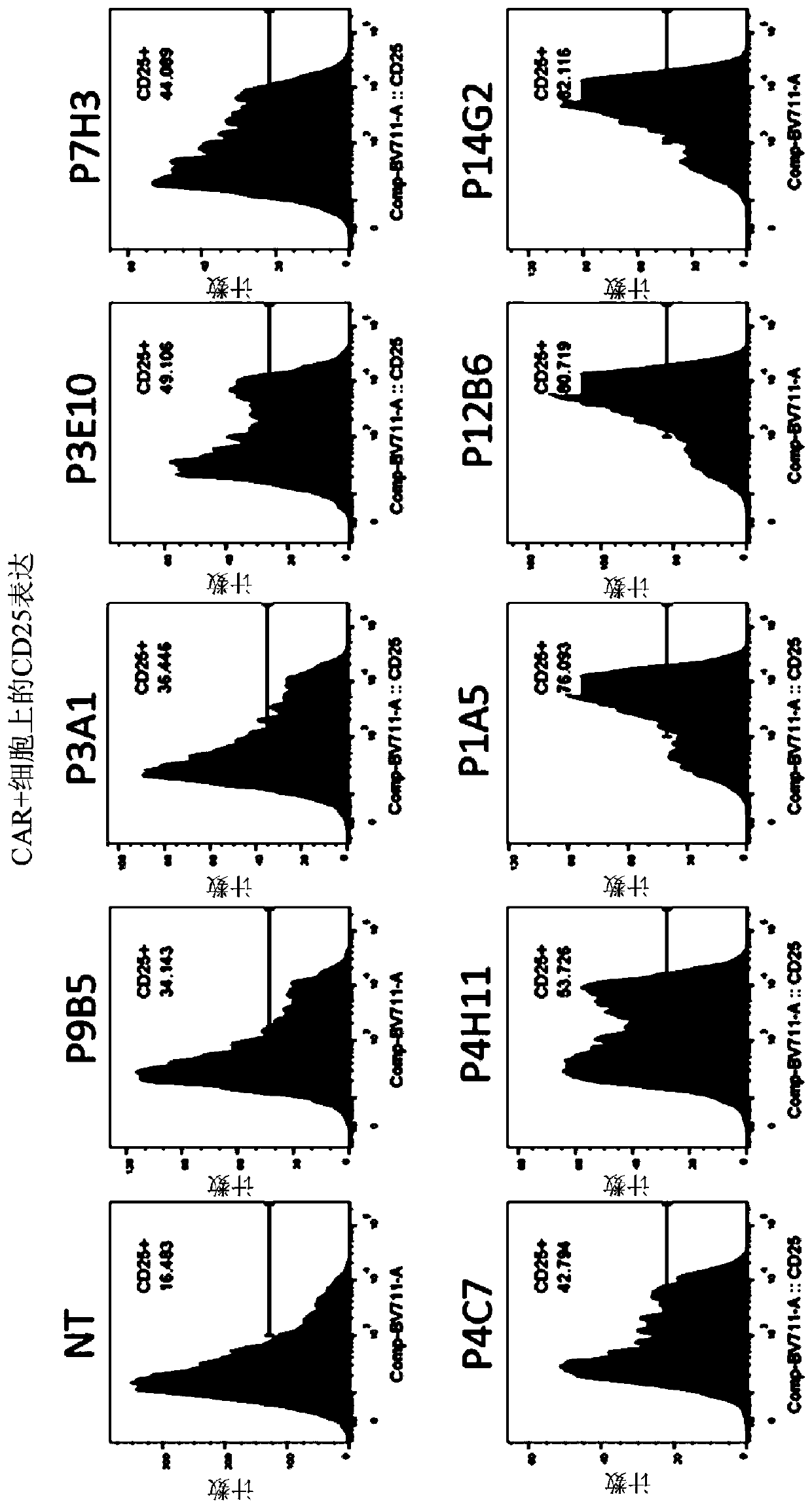
[0384] Example 2: Generation of FLT3-specific CAR-T cells
[0385] a) Plasmid
[0386] The following codon-optimized FLT3 CAR sequences listed in Table 8 below were synthesized and subcloned using EcoRI (5') and MluI (3') restriction sites in e.g. pLVX EF1a-IRES-Puro (Clontech) ) in a lentiviral vector (thus removing the IRES-Puro cassette).
[0387] Table 8: Exemplary FLT3-specific CARs
[0388]
[0389]
[0390]
[0391]
[0392] In some experiments, the sequence encoding the CAR was preceded by a sequence encoding a fluorescent protein such as blue fluorescent protein (BFP) and separated from the sequence by a sequence encoding a self-cleaving 2A peptide. Lentiviruses were generated using psPAX2, an HIV-1 gag-pol packaging plasmid, and pMD2.G, a VSV-G expression plasmid.
[0393] b) T cell activation and lentiviral transduction
[0394] Unaffected T cells were isolated from human peripheral blood mononuclear cells using the Pan T Cell Isolation Kit (Mi...
example 3
[0406] Example 3: FLT3-specific CAR expressing CD20 epitope Exhaustion of T cells
[0407] a) FLT3-specific CAR T cells expressing the CD20 epitope are sensitive to complement-dependent cytotoxicity (CDC) in the presence of rituximab
[0408] The sensitivity of FLT3-specific CAR T cells to complement was assessed in vitro using the CDC assay in the presence of anti-CD20 antibody. For this experiment, T cells transduced with the lentiviral construct to express the FLT3 CAR containing the CD20 epitope in the ectodomain were supplemented with 25% complement in the presence or absence of rituximab (100 μg / mL). (AbD serotec) mixed and incubated at 37°C and 5% CO 2 Incubate for 4 hours. Depletion of FLT3 CAR-T cells was determined by flow cytometry analysis using biotinylated FLT3 protein. Image 6 showed that cells expressing the FLT3 CAR and CD20 epitope were efficiently depleted in the presence of antibody and complement, while control FLT3-specific CAR T cells not express...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com