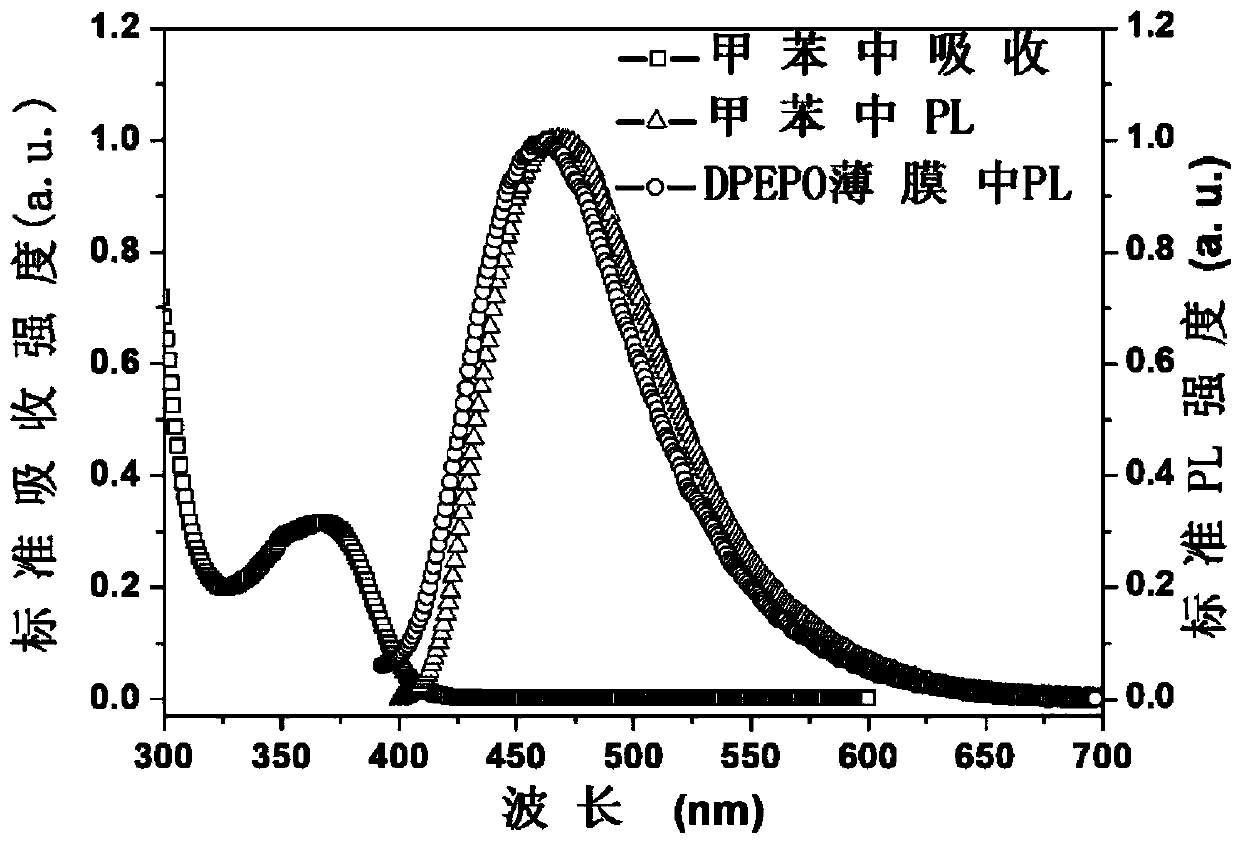
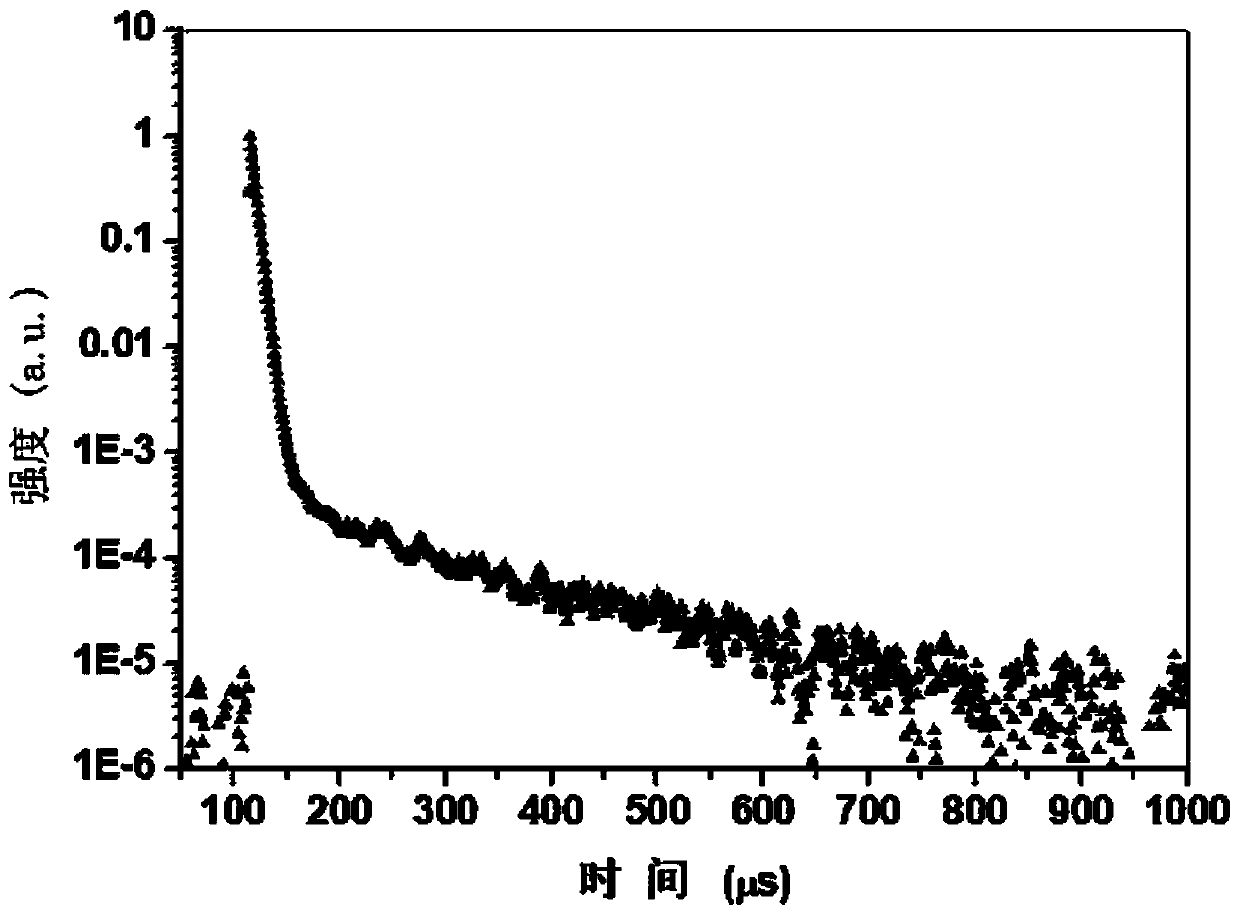
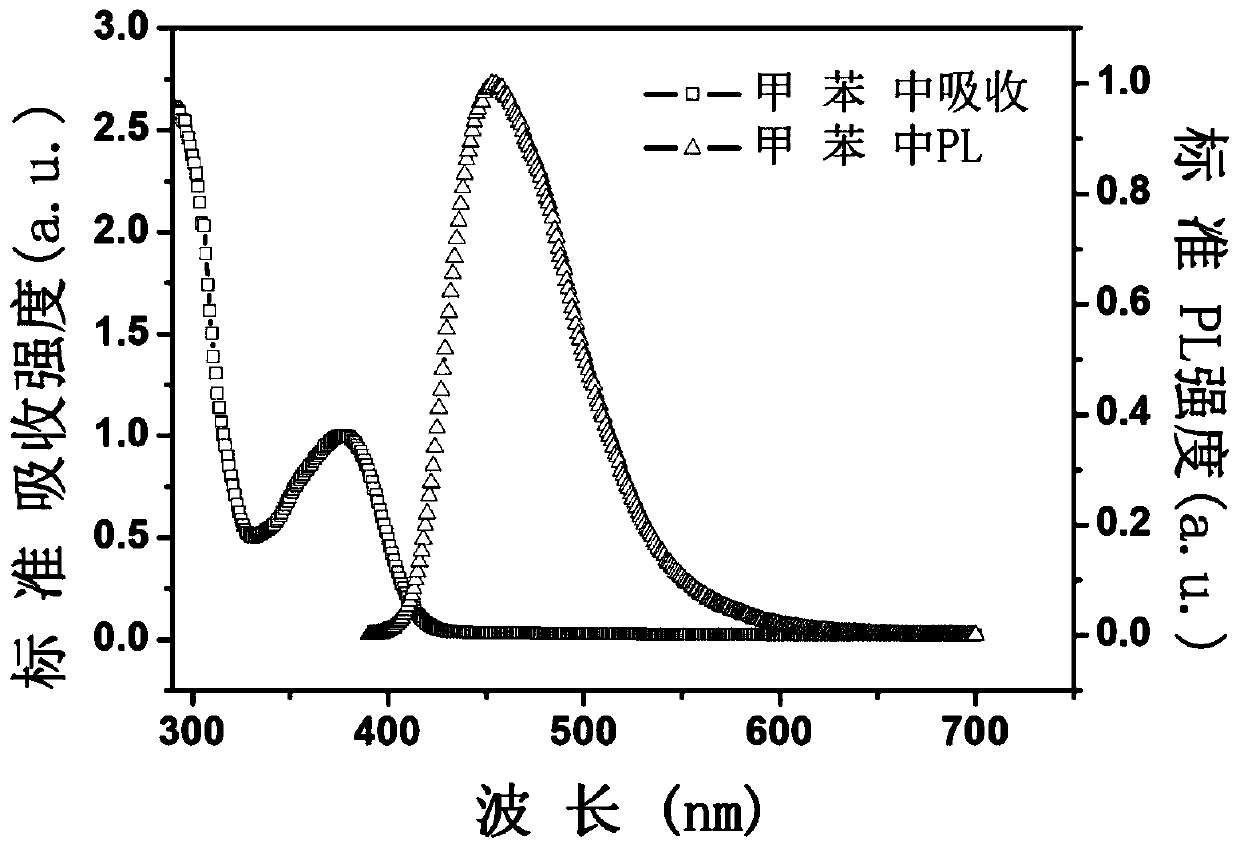
Thermal-active delayed fluorescence material and organic light-emitting element
A technology of delayed fluorescence and thermal activity, which is applied in the field of fluorescent materials and light-emitting elements, can solve problems such as the chemical stability of indole groups, and achieve high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049]
[0050] Synthetic steps:
[0051] 1. Synthesis of Intermediate I:
[0052] 5-Dimethylbromoindole (1.56g 7mmol), iodobenzene (0.56ml 5mmol), CuI (0.191g 1mmol), CsCO3 (3.26g 10mmol) were placed in a 25ml three-necked reaction flask to replace nitrogen, and 15ml ultra-dry DMF was added , replacing nitrogen. Reaction at 120°C for 16h.
[0053] Post-processing:
[0054] 1. Point board monitoring;
[0055] 2. Concentrate to dryness, dissolve in DCM, filter, wash the filter cake with DCM, concentrate the filtrate, and mix with silica gel;
[0056] 3. The petroleum ether system was passed through the column to obtain 0.77g of intermediate I, with a yield of 40%;
[0057] 4. Zoom in 3 times and re-cast.
[0058] Two, the synthesis of intermediate II
[0059] 1. Intermediate I (0.56g 1.9mmol), 3-boronate carbazole (0.61g 2.09mmol), potassium carbonate (0.79g5.7mmol), tetrakistriphenylphosphine palladium (0.11g 0.1mmol), THF 15ml, Put 7ml of water in a 100ml three-nec...
Embodiment 2
[0075]
[0076] Synthetic steps:
[0077] 1. Synthesis of Intermediate I:
[0078] 5-Bromoindole (1.36g 7mmol), iodobenzene (0.56ml 5mmol), CuI (0.191g 1mmol), CsCO3 (3.26g 10mmol) were placed in a 25ml three-necked reaction flask to replace nitrogen, and 15ml ultra-dry DMF was added to replace nitrogen . Reaction at 120°C for 16h.
[0079] Post-processing:
[0080] 1. Point board monitoring;
[0081] 2. Concentrate to dryness, dissolve in DCM, filter, wash the filter cake with DCM, concentrate the filtrate, and mix with silica gel;
[0082] 3. The petroleum ether system was passed through the column to obtain 0.68g of intermediate I, with a yield of 38%;
[0083] 4. Zoom in 3 times and re-cast.
[0084] Two, the synthesis of intermediate II
[0085] 1. Intermediate I (0.52g 1.9mmol), 3-boronate carbazole (0.61g 2.09mmol), potassium carbonate (0.79g5.7mmol), tetrakistriphenylphosphine palladium (0.11g 0.1mmol), THF 15ml water 7ml was placed in a 100ml three-neck bottl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com