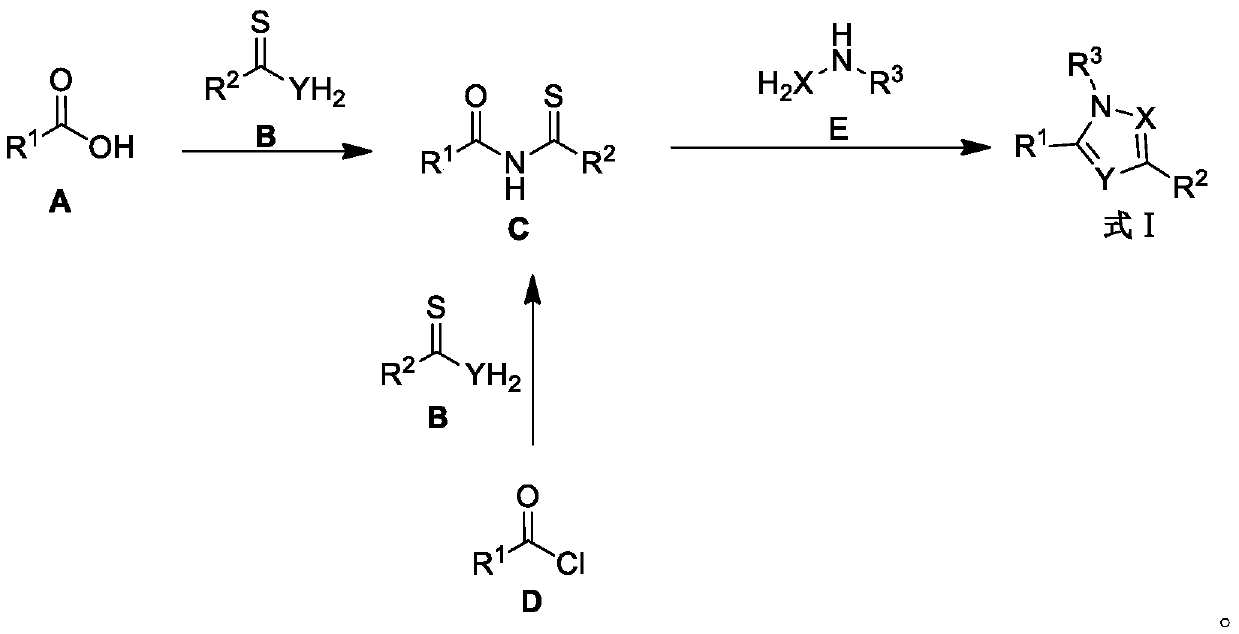
SET8 lysine methyltransferase inhibitor as well as preparation method and application thereof
A lysine methyl, inhibitor technology, applied in organic chemical methods, pharmaceutical formulations, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Preparation of 2-(3-(4-methoxybenzyl)-1-(1-methylpiperidin-4-yl)-1H-1,2,4-triazole-5-yl)? Phenyl (compound 1) hydrochloride
[0061]
[0062] Step 1: Preparation of tert-butyl 2-((2-(4-methoxyphenyl)thioacetyl)carbamoyl)morpholine-4-carboxylate (compound 1-3)
[0063] The reaction equation is as follows:
[0064]
[0065] 4-(tert-butoxycarbonyl)morpholine-2-carboxylic acid (1.0mmol, 231mg), 2-(7-oxybenzotriazole)-N,N,N' shown in formula 1-1 , N'-tetramethyluronium hexafluorophosphate (HATU, 1.1mmol, 418mg) was dissolved in 4mL of dry dichloromethane (DCM), under nitrogen protection, stirred at room temperature for 20 minutes, and then added formula 1 to the above system 2-(4-Methoxyphenyl)thioacetamide (1.1mmol, 199mg) shown in -2, after stirring for 1 hour, N,N-diisopropylethylamine (DIEA, 1.1mmol, 142 mg) was dropped into the above reaction system, and continued to stir and react under nitrogen protection at room temperature for 2 days; the reaction...
Embodiment 2
[0075] Example 2 Preparation of 2-(1,3-bis(4-methoxybenzyl)-1H-1,2,4-triazol-5-yl)morpholine (compound 2)
[0076]
[0077] Step 1: Preparation of tert-butyl 2-(1,3-bis(4-methoxybenzyl)-1H-1,2,4-triazol-5-yl)morpholine-4-carboxylate (Compound 2 -1)
[0078]
[0079] Compound 1-3 (0.15mmol, 59mg), (4-methoxybenzyl)hydrazine hydrochloride (0.18mmol, 34mg), sodium acetate (0.36mmol, 30mg) were successively dissolved in 1mL acetic acid and 1mL 1,4 - After being placed in a mixed solvent of dioxane, seal the sealant, and then react under heating at 80° C. until the compound 1-3 is completely reacted. After the reaction solution was diluted with 30mL ethyl acetate, washed successively with 20mL saturated sodium carbonate solution and saturated sodium chloride solution, dried over anhydrous sodium sulfate, concentrated, and separated by column chromatography [V (petroleum ether): V (ethyl acetate )=3:1-1:1], 50 mg of light yellow oil 2-1 was obtained, and the yield was 68%. ...
Embodiment 3
[0084] Example 3 Preparation of 2-(1-cyclohexyl-3-(4-methoxybenzyl)-1H-1,2,4-triazol-5-yl)morpholine (compound 3)
[0085]
[0086] Step 1: Preparation of tert-butyl 2-(1-cyclohexyl-3-(4-methoxybenzyl)-1H-1,2,4-triazol-5-yl)morpholine-4-carboxylate ( Compound 3-1)
[0087]
[0088] Compound 1-3 (0.16mmol, 63mg), cyclohexylhydrazine hydrochloride (0.2mmol, 30mg), sodium acetate (0.36mmol, 30mg) were successively dissolved in a mixture of 1mL acetic acid and 1mL 1,4-dioxane After being placed in the solvent, the sealant was sealed, and then the reaction compound 1-3 was completely reacted under heating at 80°C. After the reaction solution was diluted with 30mL ethyl acetate, washed successively with 20mL saturated sodium carbonate solution and saturated sodium chloride solution, dried over anhydrous sodium sulfate, after concentrating the reaction solution, column chromatography separated [V (petroleum ether): V( Ethyl acetate)=3:1-1:1], 40mg of light yellow oily substan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com